MBIO 2700 / Topic 7a: Monosaccharides
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
25 Terms
What are the three key functions of carbs?
> Transporting + storing energy.
> Structural integrity.
> Providing info.
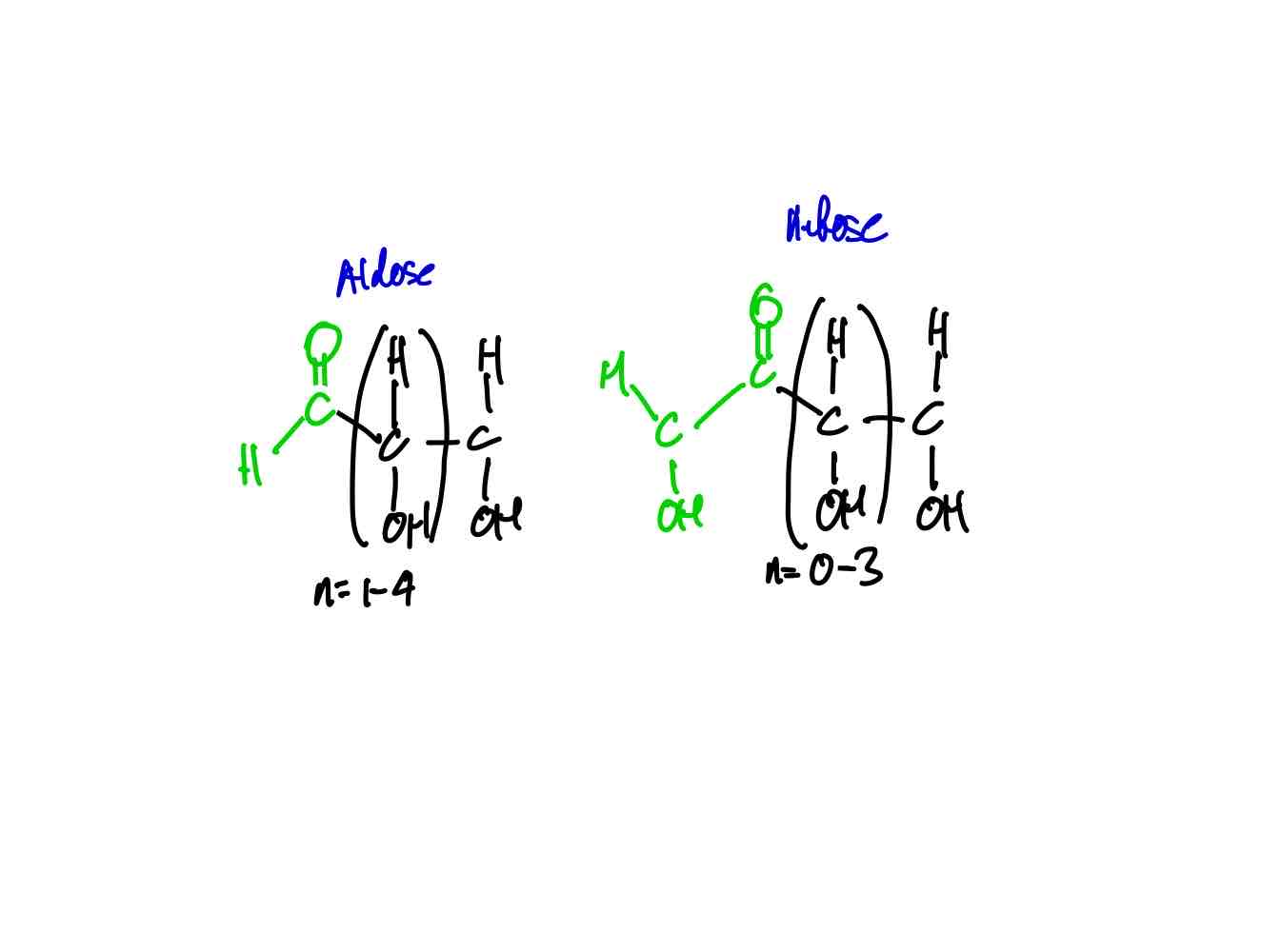
What are the two major carb families? Draw their generic structure.
Aldoses and Ketoses.
What is the chemical formula for a carbohydrate?
Cm(H2O)n
Differentiate the following terms: saccharides, monosaccharides, disaccharides, oligosaccharides, polysaccharides.
> Saccharides = Another scientific term for sugars.
> Monosaccharides = One sugar unit.
> Disaccharides = Two sugar units.
> Oligosaccharides = Several sugar units.
> Polysaccharides = Long chains of hundreds to thousands of sugar units.
What’s so special about the naming scheme of 4C and 5C ketoses?
By convention, ketotetroses and ketopentoses are named after aldotetroses and aldopentoses but with the inclusion of “ul.” E.g., one of the 5C aldoses is named ribose. Its 5C ketose counterpart is named rib-UL-ose.
Abbreviate the following: glucose, glucosamine, fructose, galactose, galactosamine, N-acetylglucosamine, and ribose.
> Glc.
> GlcN.
> Fru.
> Gal.
> GalN.
> GlcNAc.
> Rib.
What do aldoses and ketoses have in respect to chirality?
All aldoses and ketoses except the ketose dihydroxyacetone have chiral carbons.
How do you assign stereochemical configuration to a sugar?
> If chiral C furthest from CO has -OH on right → D.
> If chiral C furthest from CO has -OH on left → L.
+ Dextrorotatory (+) is the direction by which they rotate plane-polarized light.
+ Levorotatory (-) is the direction by which they rotate plane-polarized light.
Naturally and mostly, in what stereochemical configuration are sugar?
D is the usual stereochemical configuration for sugars.
What are epimers?
Diastereomers specifically differing in configuration at only one chiral carbon.
How does a hemiacetal look like? How does a hemiketal look like?
> Hemiacetal = central C bonded to -OH, -OR, -H, and -R
> Hemiketal = central C bonded to -OH, -OR, -R, and -R’
Why do carbohydrates cyclize?
> Sugars have both OH groups and a CO group.
> OH and CO can react irreversibly intramolecularly → cyclization.
Why is ring strain a limiting factor in sugar cyclization?
> Sugar rings have bond angles < ideal (109.5°).
> Poor orbital overlap → weaker σ bonds.
> Increases ring strain, making some ring sizes less stable.
What’s the relationship of isomers differing only in their configuration about their hemiacetal/hemiketal carbon atom in cyclic sugars? What do you call the carbonyl carbon in this case?
Anomers, with the CO carbon atom being called the anomeric carbon.
In regards to stereochemistry, what does cyclization lead to?
> O of OH attacks C of CO.
> Attack can occur from the top or bottom face.
> Forms two anomers from two diff scenarios:
• Top face attack → OH ends up down → α-anomer = OH is anti to CH₂OH (last carbon)
• Bottom-face attack → OH ends up up → β-anomer = OH is syn to CH₂OH (last carbon)
From a Haworth diagram, what is top and bottom in respect to Fischer projections?
> Top face in Haworth is left in Fischer.
> Bottom face in Haworth is right in Fischer.
> What is a six-membered sugar cyclic system called? Why?
> What is a five-membered sugar cyclic system called? Why?
> Pyranose because they resemble the molecule pyran.
> Furanose because they resemble the molecule furan.
What is mutarotation? How is mutarotation possible?
> Interconversion between α and β.
> Only possible by trace amounts of its linear form in solution.
All α, β, and linear forms exist in equilibrium with each other.
What are the two conformations that α and β can take? Which is predominant?
> Each α + each β can take on two conformations: chair + boat.
> Chair = more stable b/c bow + stern of boat can sterically + torsionally clash.
What is the more predominant form: α or β? Why?
> In both chair + boat conformations of α, you have a relatively bulky OH in an axial position → 1,3-diaxial interactions w/ H.
> In one of the chair conformations of β, you have a conformation wherein all bulky OH groups are equatorial → no 1,3-diaxial interactions.
> β is predominant due to sterics.
What are the six key derivative classes sugars?
> Reduced sugars = C=O reduced to C-OH.
> Reducing sugars = oxidized to aldonic acids (sugar + COOH).
> Phosphate sugars = OH replaced w/ PO43−.
> Amino sugars = OH replaced w/ NH2.
> Amide sugars = OH replaced w/ amide.
> Deoxy-sugars = OH replaced w/ H.
How do reducing sugars reduce/do their job?
> In the conversion of aldose → aldonic acid, an electrons are given up to reduce metal ions, e.g. Cu2+ + 1e- → Cu+.
> Test if Cu can be seen as precipitate/orange colour change.
Reducing sugars must be in two forms. What are these two forms, and why?
Linear form
> Cyclic → locked in hemiacetal/hemiketal = no CO.
> Only linear form exposes CO.
> CO needed for redox.
Aldose form
> Aldoses = free CHO group → oxidized to COOH.
> Ketoses = must isomerize first to aldose → free CHO group → oxidized to COOH.
> Regardless, in the end, you need aldose form.
Ketoses tautomerize → enediol int → aldose → aldonic acid.
How do phosphate sugars act as intermediates and prevent sugar transport?
> PO43− = charged → can’t cross lipid membranes (hydrophilic substance vs. hydrophobic tails).
> If phosphate sugar in cytosol → trapped inside cell.
> Phosphorylation marks sugars for processing by enzymes → intermediates in pathways.
Usually, which carbon is the hotspot for amination for hexoses, and why?
At C2, due to enzyme specificity.