Photosynthesis, Respiration, and Atomic Structure
1/73
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
74 Terms
PHOTOSYNTHESIS
water + carbon dioxide → glucose + oxygen
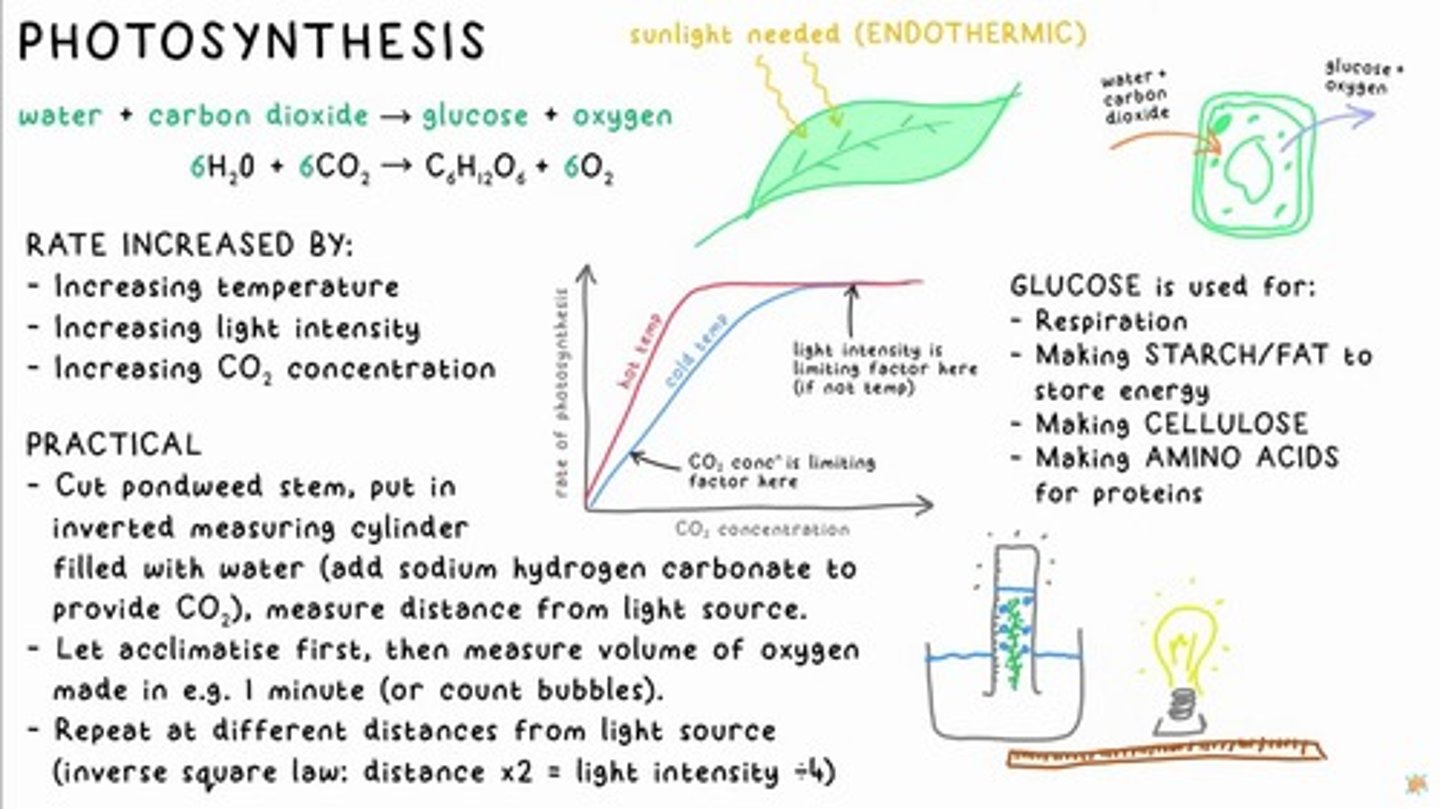
Photosynthesis Equation
6H₂O + 6CO₂ → C6H₁₂O6 + 6O₂
Factors Increasing Rate of Photosynthesis
Increasing temperature, Increasing light intensity, Increasing CO₂ concentration
Limiting Factor in Photosynthesis
Light intensity is the limiting factor if temperature is not.
Practical Method for Measuring Photosynthesis
Cut pondweed stem, put in inverted measuring cylinder filled with water, measure distance from light source.
Inverse Square Law
Distance x2 = light intensity ÷ 4
Uses of Glucose
Respiration, Making starch/fat to store energy, Making cellulose, Making amino acids for proteins.
AEROBIC RESPIRATION
glucose + oxygen → water + carbon dioxide
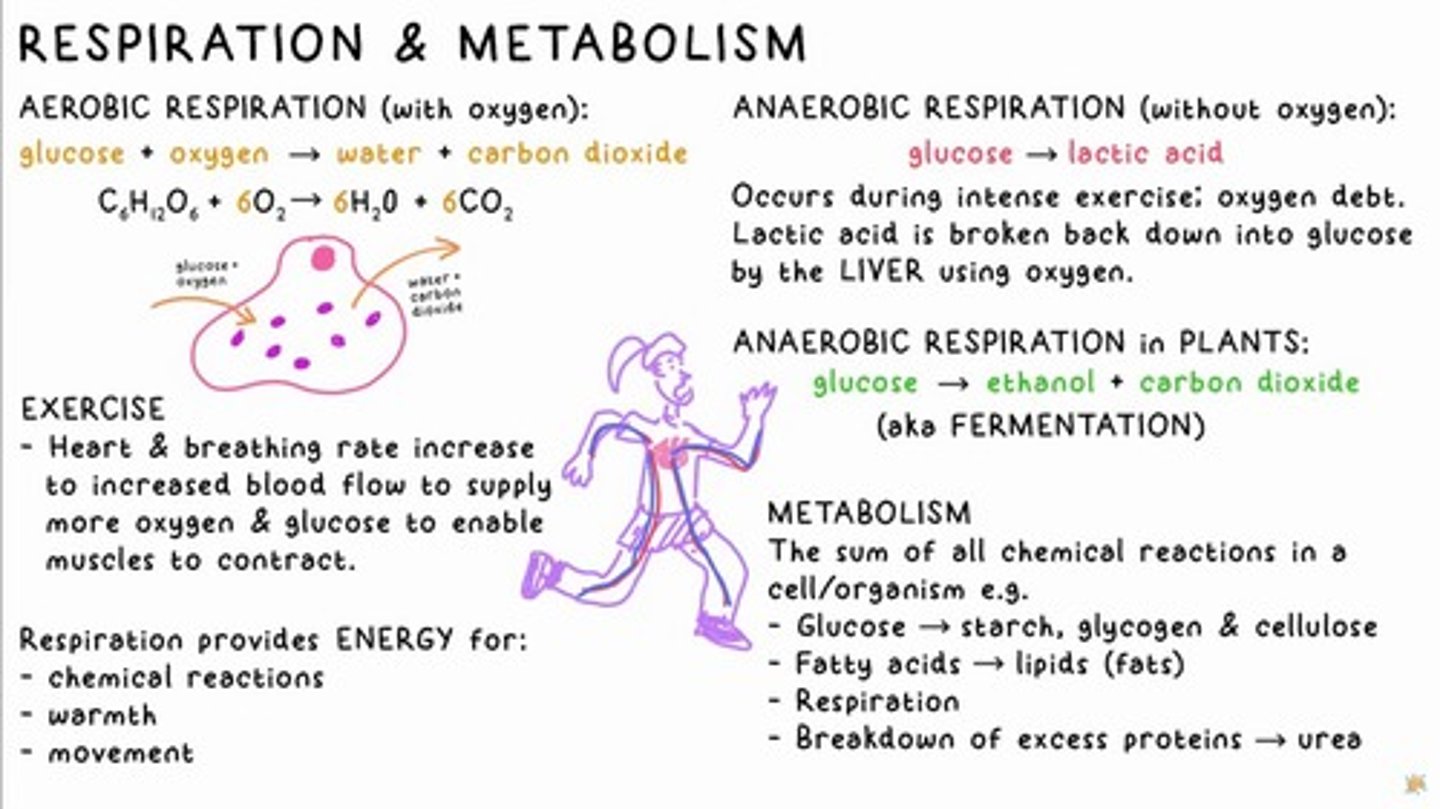

Anaerobic Respiration
glucose → lactic acid
Oxygen Debt
Occurs during intense exercise when lactic acid is broken down into glucose by the liver using oxygen.
Anaerobic Respiration in Plants
glucose → ethanol + carbon dioxide (aka fermentation)
METABOLISM
The sum of all chemical reactions in a cell/organism.
METALLIC BONDING
How metal atoms bond to each other forming a lattice of ions surrounded by a sea of delocalised electrons.
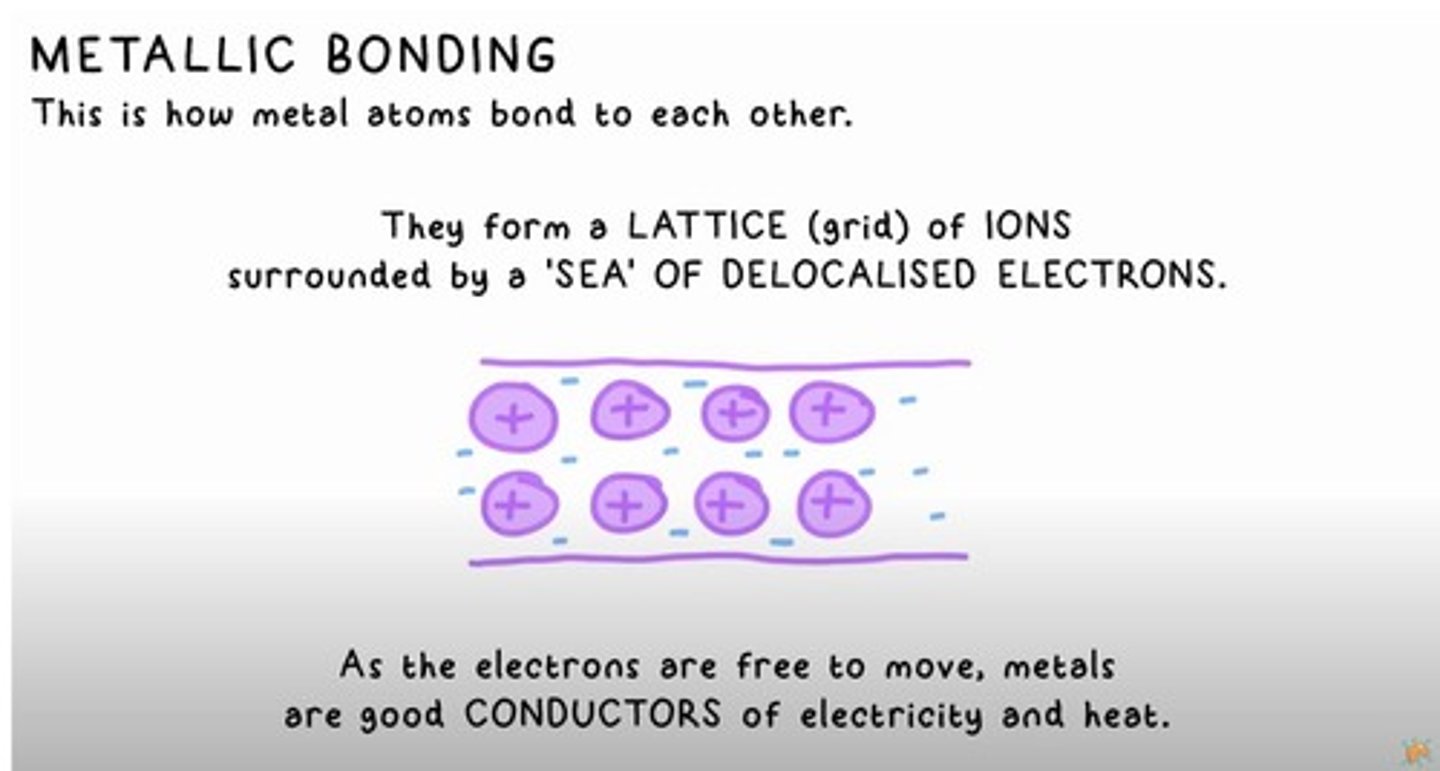
Conductivity of Metals
Metals are good conductors of electricity and heat due to free-moving electrons.
IONIC BONDING
How metals bond to non-metals by donating electrons to form ions.
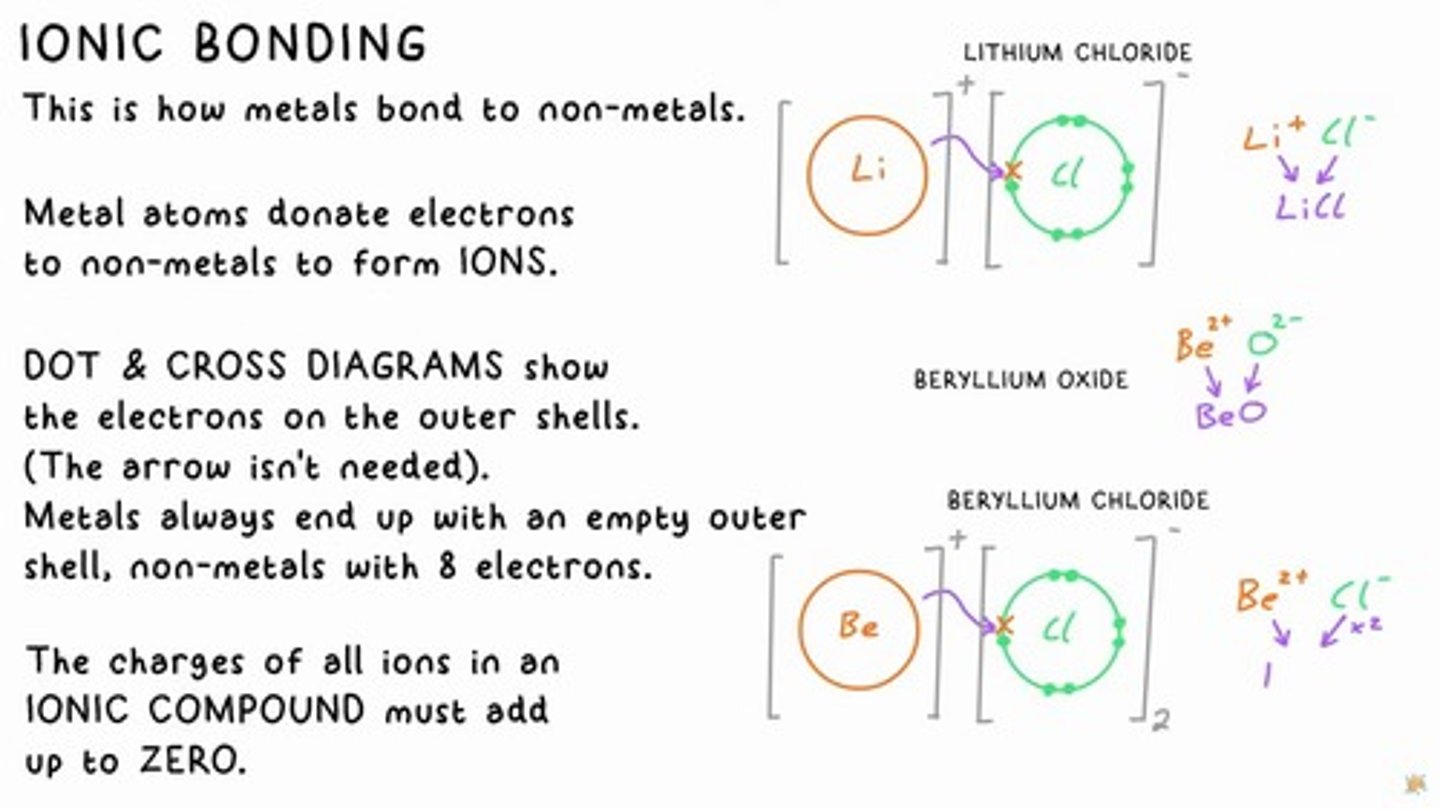
Ionic Compounds
The charges of all ions in an ionic compound must add up to zero.
IONIC STRUCTURES
Ions are arranged in a lattice of repeating units of positive and negative ions forming a crystal.
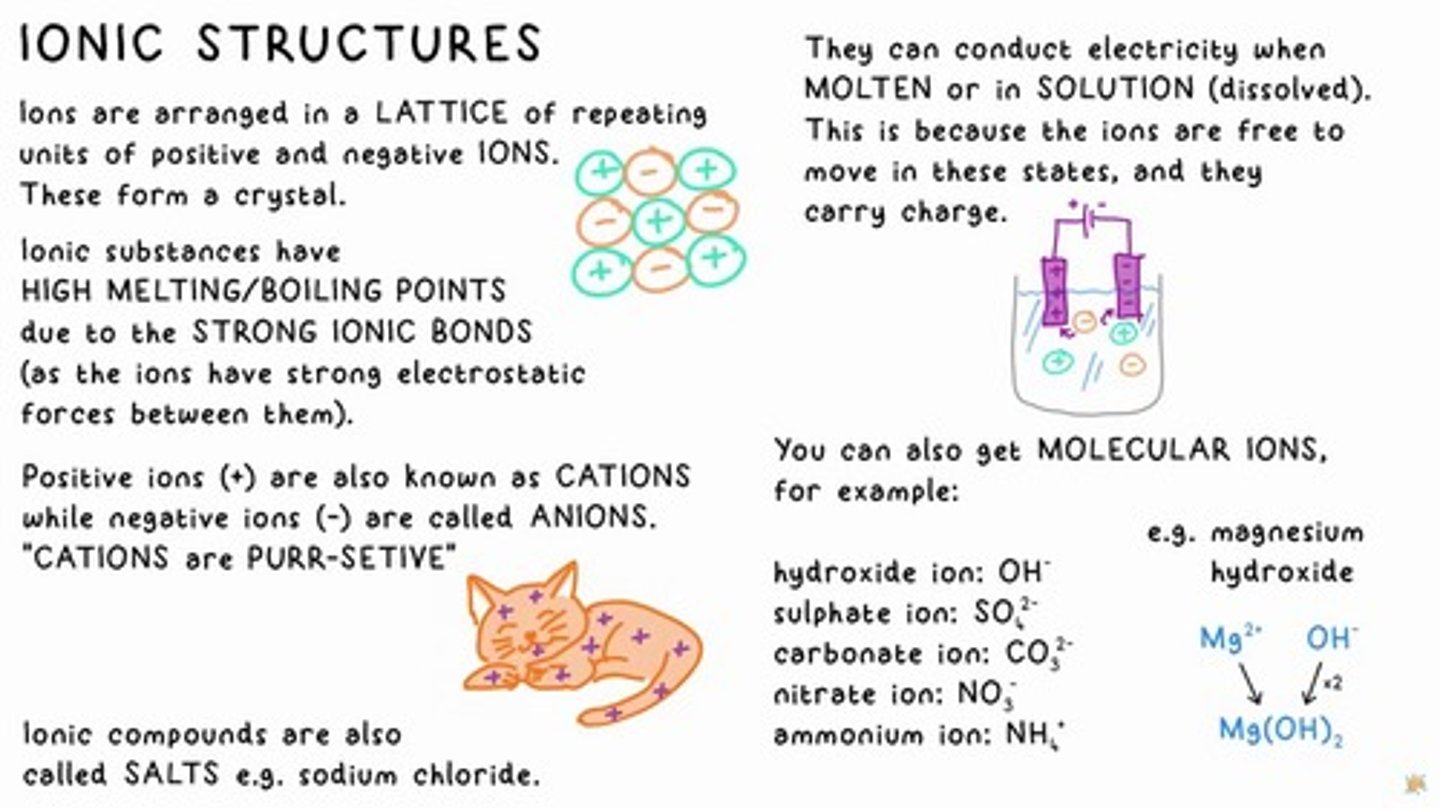
Melting/Boiling Points of Ionic Compounds
Ionic substances have high melting/boiling points due to strong ionic bonds.
CATIONS
Positive ions.
ANIONS
Negative ions.
Conductivity of Ionic Compounds
Ionic compounds can conduct electricity when molten or in solution.
SALTS
Ionic compounds are also called salts, e.g., sodium chloride.
hydroxide ion
OH-
sulphate ion
SO²-
carbonate ion
CO3²-
nitrate ion
NO₂
ammonium ion
NH
covalent bonding
Atoms SHARE electrons to gain FULL OUTER SHELLS.
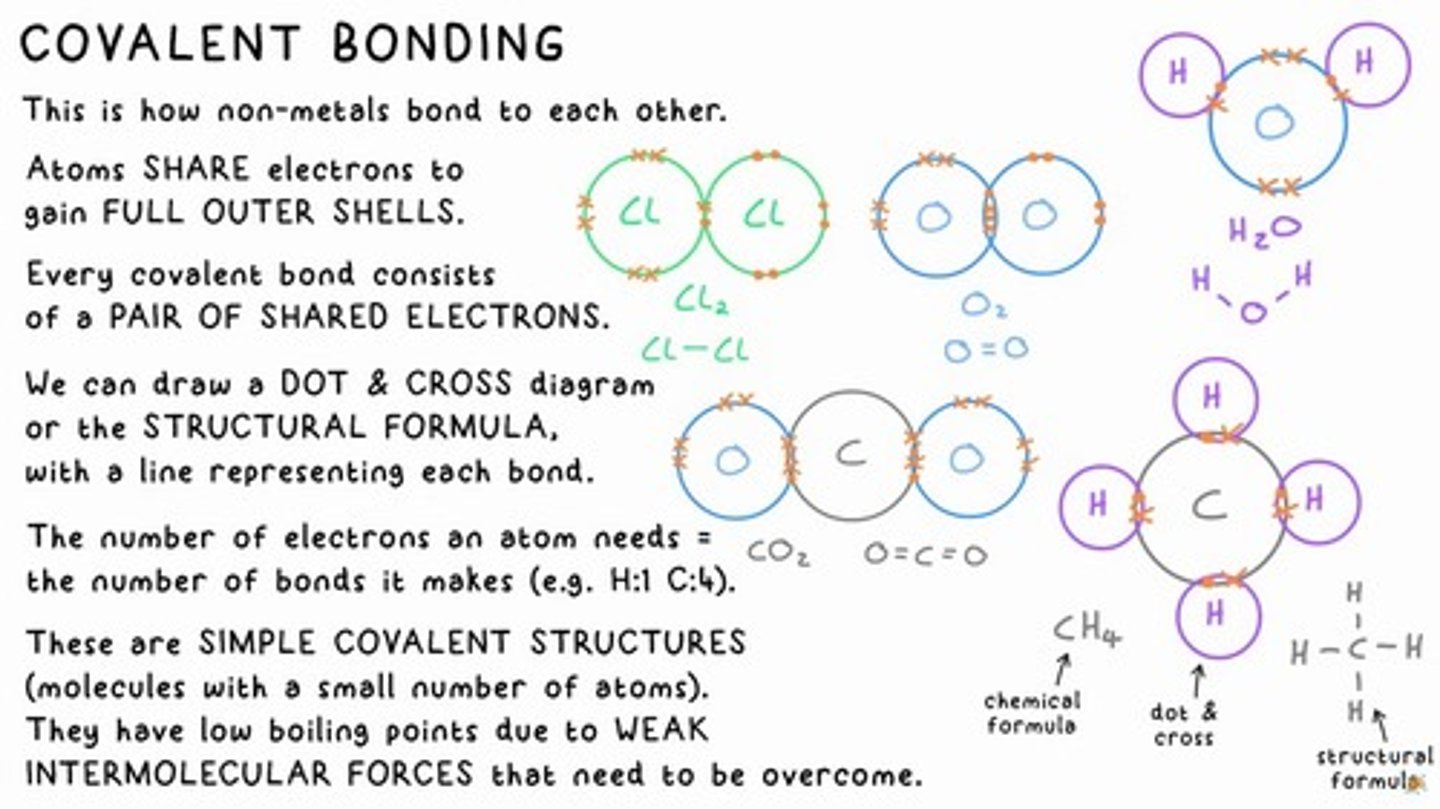

covalent bond
Every covalent bond consists of a PAIR OF SHARED ELECTRONS.
DOT & CROSS diagram
A representation of covalent bonding showing shared electrons.
structural formula
A diagram with a line representing each bond.
simple covalent structures
Molecules with a small number of atoms that have low boiling points due to WEAK INTERMOLECULAR FORCES.
allotropes of carbon
Structures made of the same element but arranged differently, resulting in giant molecules.
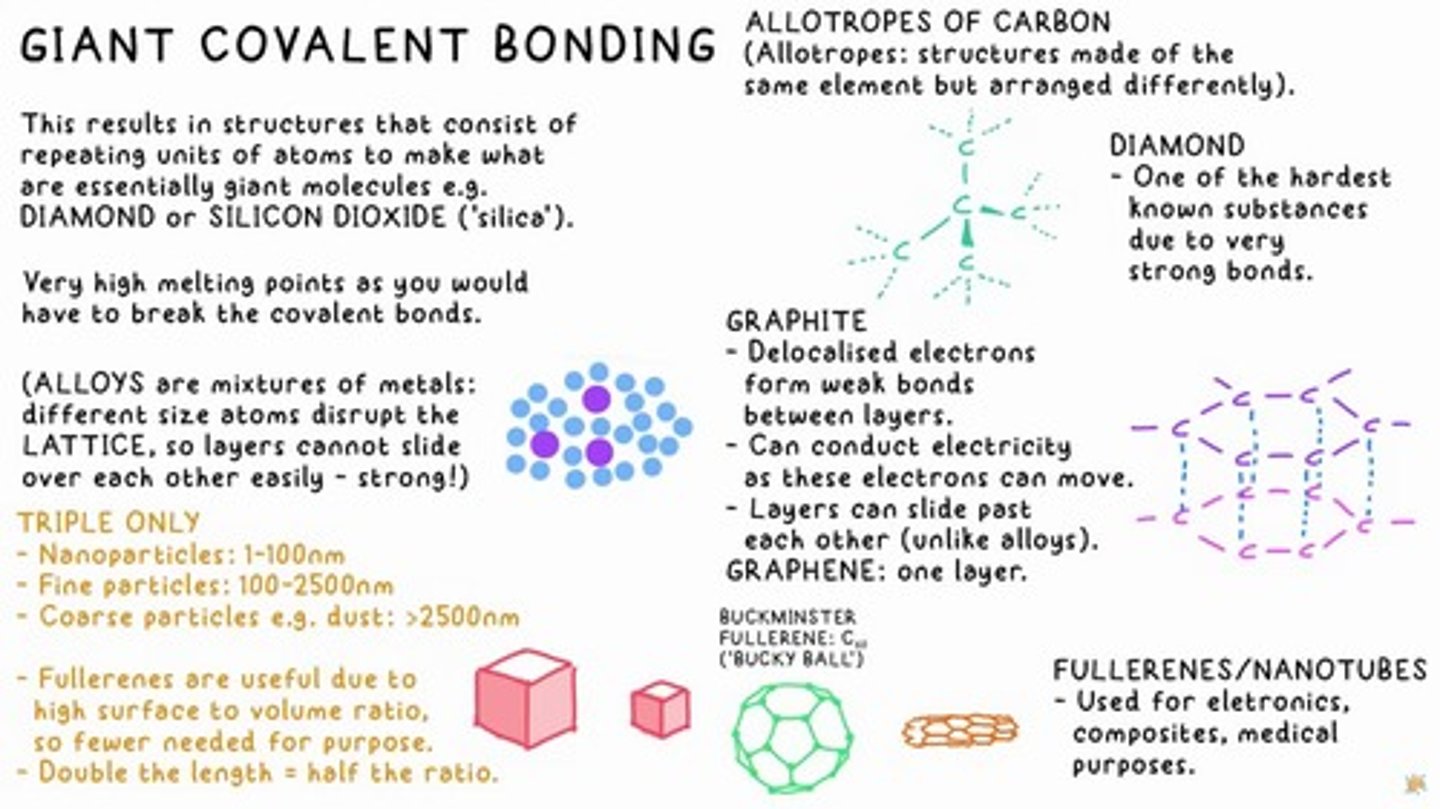

diamond
One of the hardest known substances due to very strong bonds.
silicon dioxide
Also known as 'silica', a giant covalent structure.
fullerenes
Molecules with high surface to volume ratio, useful for various applications.
graphite
Contains delocalised electrons and can conduct electricity.
Buckminster fullerene
C60, also known as 'Bucky Ball'.
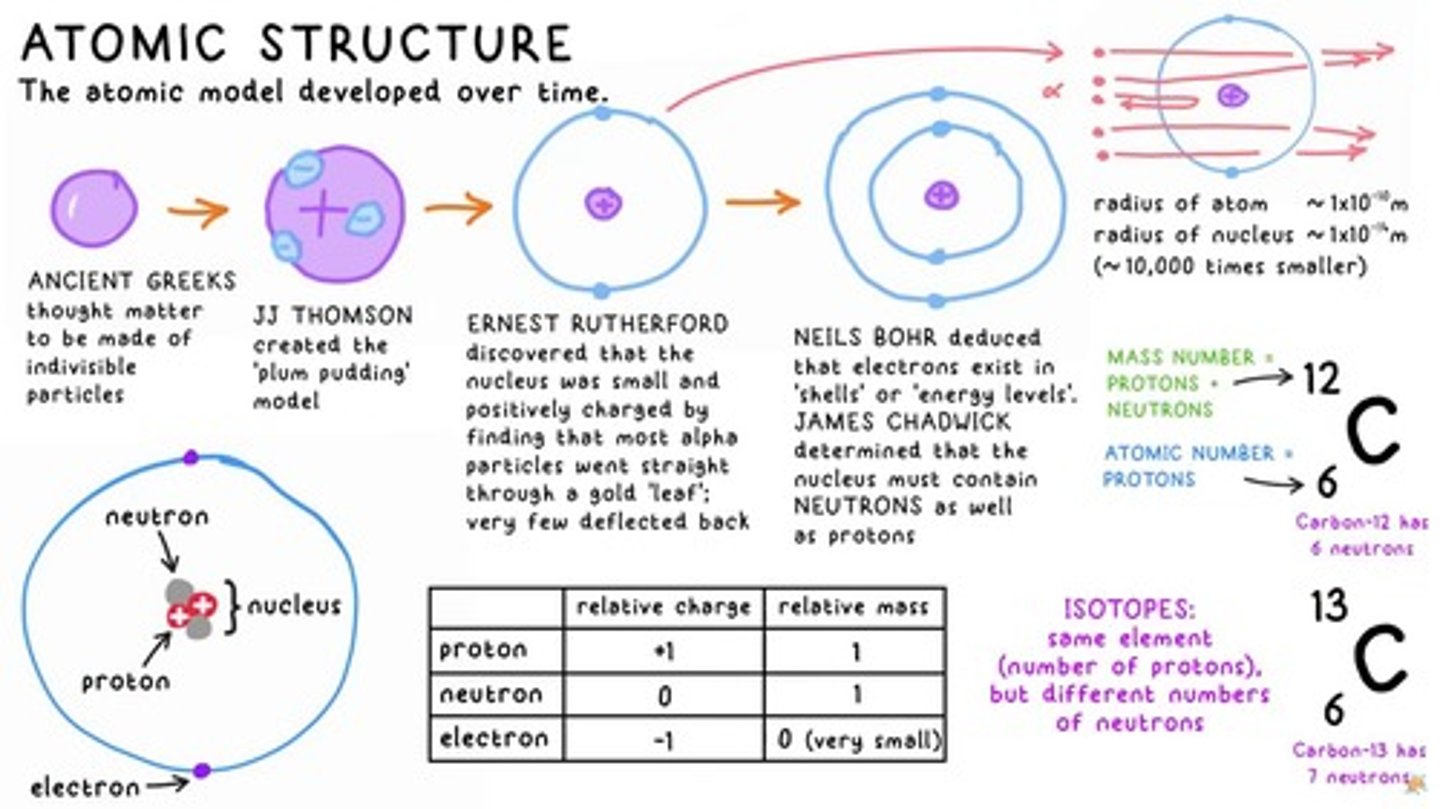
atomic structure
The model of matter developed over time, including protons, neutrons, and electrons.
mass number
The sum of PROTONS and NEUTRONS in an atom.
atomic number
The number of PROTONS in an atom.
isotopes
Atoms of the same element with different numbers of neutrons.

nuclear decay equations
Equations describing the process where unstable nuclei emit radiation to become more stable.
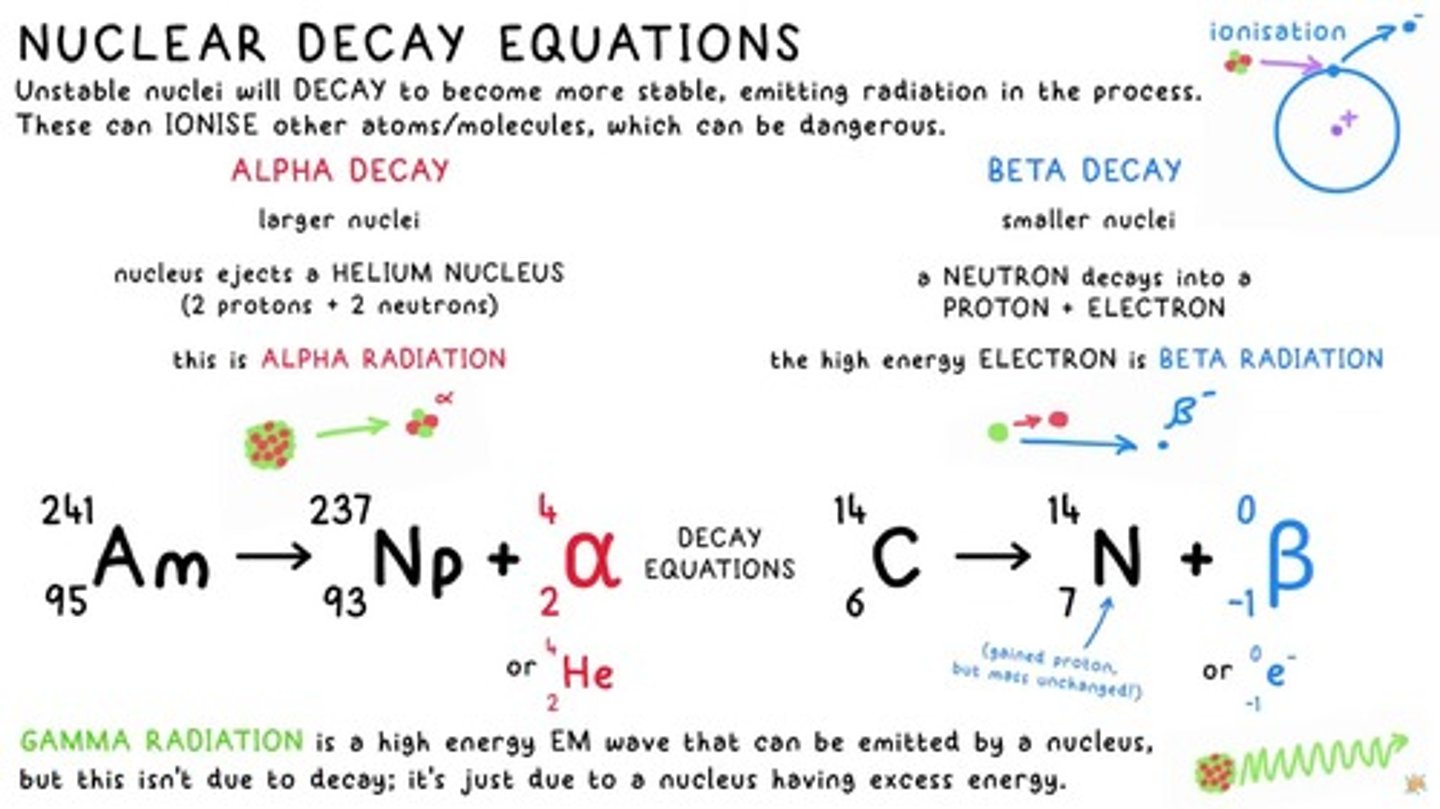

Alpha Decay
Larger nuclei nucleus ejects a HELIUM NUCLEUS (2 protons + 2 neutrons).
Alpha Radiation
This is ALPHA RADIATION.

Beta Radiation
The high energy ELECTRON is BETA RADIATION.
Beta Decay
Smaller nuclei a NEUTRON decays into a PROTON ELECTRON.
Gamma Radiation
GAMMA RADIATION is a high energy EM wave that can be emitted by a nucleus, but this isn't due to decay; it's just due to a nucleus having excess energy.
Radioactivity
Radioactivity (or just 'activity') is the rate of decay in a sample of radioactive material.
Half-Life
The TIME it takes for the activity to halve; this is also true for NUMBER OF UNSTABLE NUCLEI LEFT, and also MASS.
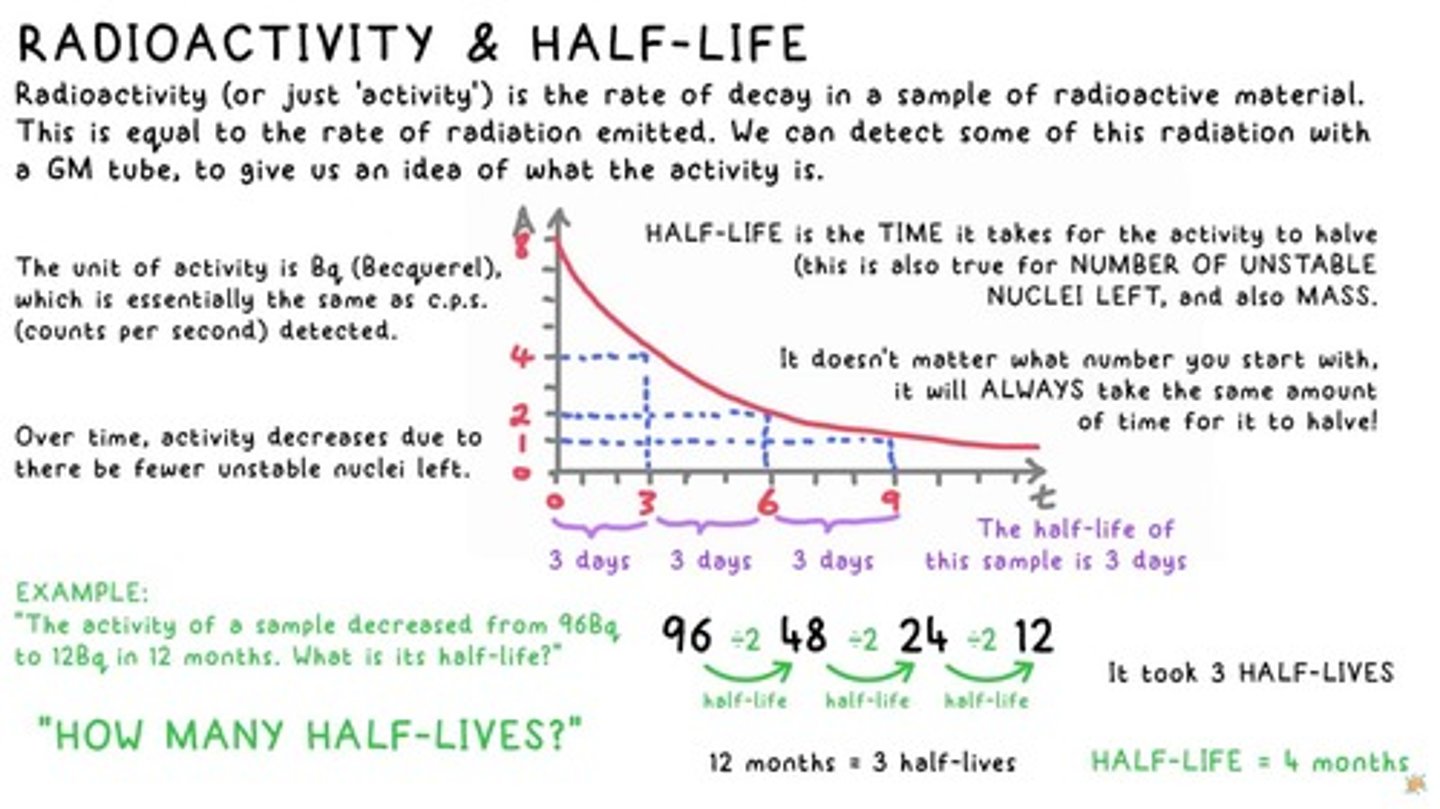

Activity Unit
The unit of activity is Bq (Becquerel), which is essentially the same as c.p.s. (counts per second) detected.
Half-Life Example
The activity of a sample decreased from 96Bq to 12Bq in 12 months. What is its half-life?
Half-Life Calculation
12 months = 3 half-lives. It took 3 HALF-LIVES.
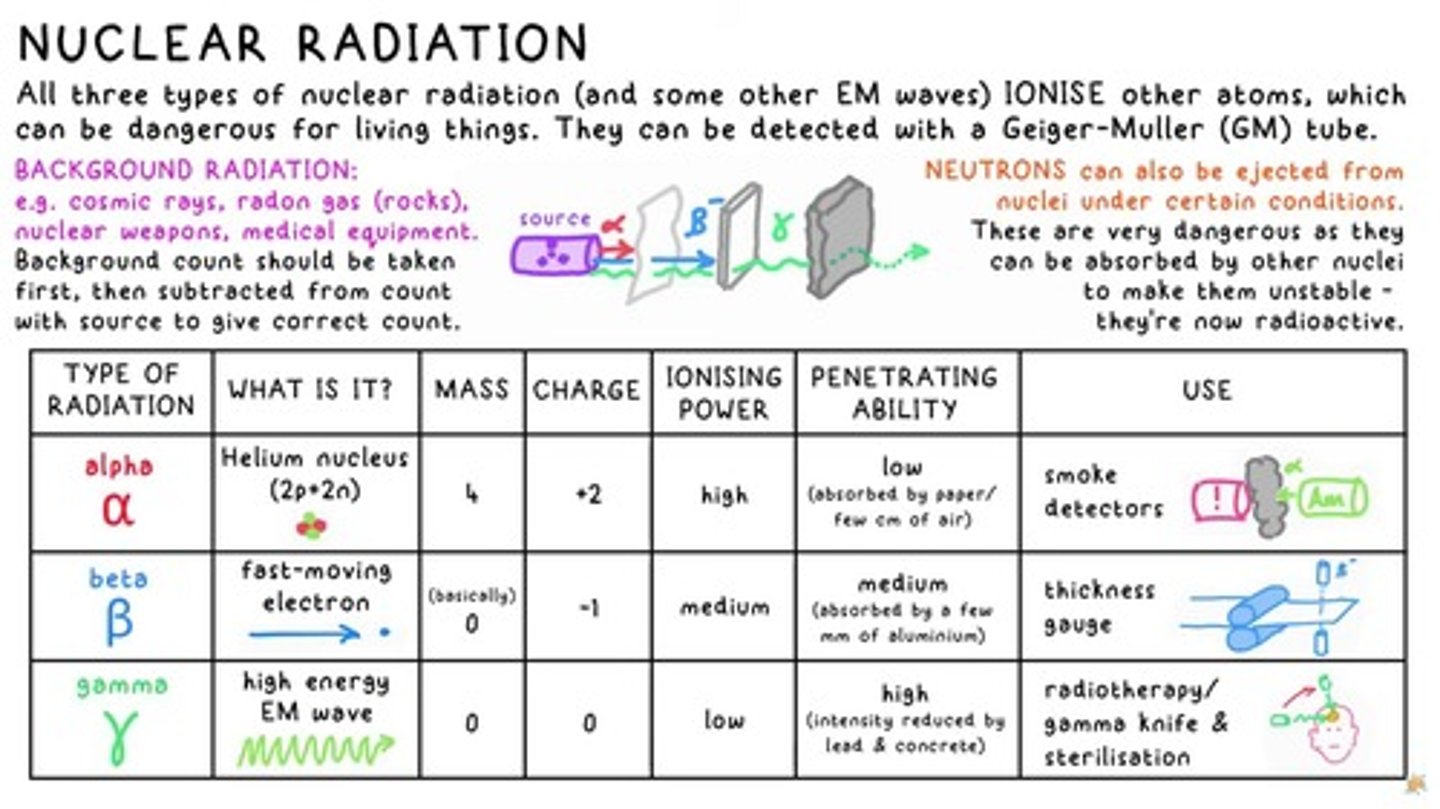
Ionising Power of Radiation
All three types of nuclear radiation IONISE other atoms, which can be dangerous for living things.
Background Radiation
e.g. cosmic rays, radon gas (rocks), nuclear weapons, medical equipment.
Alpha Radiation Characteristics
MASS: Helium nucleus (2p+2n), CHARGE: +2, IONISING POWER: high, PENETRATING ABILITY: low.
Beta Radiation Characteristics
MASS: 0, CHARGE: -1, IONISING POWER: medium, PENETRATING ABILITY: medium.
Gamma Radiation Characteristics
MASS: 0, CHARGE: 0, IONISING POWER: low, PENETRATING ABILITY: high.
Neutrons Ejection
NEUTRONS can also be ejected from nuclei under certain conditions.
Fission
FISSION is the splitting of a (heavier) nucleus (like Uranium-235) into two 'daughter' nuclei, releasing energy.
Fusion
FUSION is the joining together of two (lighter) nuclei (such as heavy hydrogen-2, also called deuterium, into helium), releasing energy.
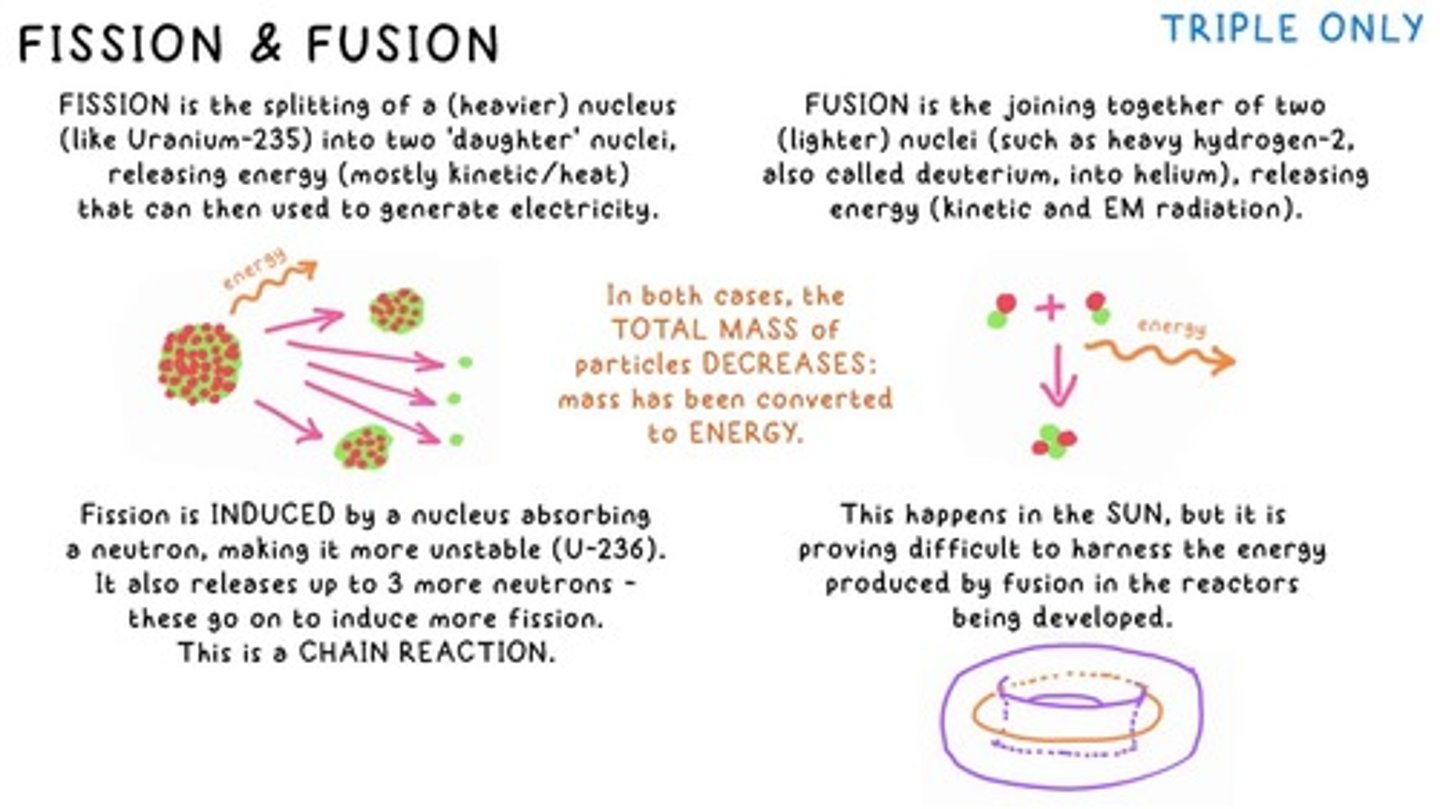

Energy Conversion in Fission and Fusion
In both cases, the TOTAL MASS of particles DECREASES: mass has been converted to ENERGY.
Induced Fission
Fission is INDUCED by a nucleus absorbing a neutron, making it more unstable (U-236).
Chain Reaction
Fission releases up to 3 more neutrons - these go on to induce more fission.
Challenges of Fusion
This happens in the SUN, but it is proving difficult to harness the energy produced by fusion in the reactors being developed.