CHEMISTRY EVERYTHING
1/223
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
224 Terms
Fuel
a substance with stored energy that can be released relatively easily for use as heat or power.
Why are H+ ions required in oxidation reactions?
Oxidation reactions using oxidising agents like MnO₄⁻ require H⁺ ions to provide hydrogen for water formation and balance oxygen atoms during reduction.
The acidic environment also increases the oxidising strength of MnO₄⁻, allowing it to be fully reduced to Mn²⁺ rather than MnO₂.
Renewable Fuels
Recourses that can be replaced by natural processes within a relatively short period of time
Examples of Renewable Fuels
Bioethanol, Biogas, Biodiesel.
Examples of Non-Renewable Fuels
Coal, Crude Oil, Natural Gas.
Fossil Fuels
Combustible blackish sedimentary rock, consisting primarily of carbon, found underneath the Earth, formed from dead plant matter that has been subjected to high temp and high pressure over millions of years.
Natural Gas
Composed of ~80% methane, ~20% other hydrocarbons, used for heating and electricity.
Fracking
the process of obtaining natural gas that is found with coal or shale deposits.
Coal Seam Gas (CSG)
Natural gas obtained from reserves found under the earth, specifically from coal or shale deposits.
Crude Oil (petroleum)
Mixture of hydrocarbons (alkanes, cycloalkanes); refined to petrol and diesel.
sustainable
the ability to meet present needs without compromising the ability of future generations to meet their own needs
Carbon Neutral
refers to the recycling of carbon, the carbon released as a greenhouse gas (CO₂) is approximately the same amount absorbed during the process of fuel creation.
Enhanced greenhouse effect
a consequence of increased greenhouse gas concentrations in the atmosphere due to human activities.
Biofuels
A renewable fuel source derived from biological matter.
Bioethanol
Produced when glucose from plant matter is fermented.
Production of bioethanol
fermentation of glucose
glucose is obtained from plants (sugarcane, wheat)
enzymes catalyse the fermentation process to produce ethanol
distillation
fermentation produces a solution that is 10%v/v ethanol (ethanol needs to be separated from water to produce a useful fuel)
solution is heated so ethanol gas (BP~78ºC) is collected top of the column while water stays at the bottom
ethanol collected and dehydrating agents are used to remove remaining traces of water
REQUIRES SIGNIFICANT QUANTITY OF ENERGY TO BOIL → not carbon neutral
%v/v
refers to how much of a particular liquid is dissolved in a 100 mL solution.
Biogas
Combustible, renewable fuel produced when animal waste or other organic material rots in the absence of oxygen.
consisting mainly of CH4 and CO2
Biodiesel
Derived from triglycerides in vegetable oils or animal fats via transesterification.
Viscosity
The measure of a fluid’s resistance to flow
Photosynthesis
The chemical process undertaken by plants to convert light energy into chemical energy.
endothermic
Cellular Respiration
The process of using glucose produced by plants to produce energy in the cells of organisms.
Glucose
One of the main fuels that is used to power the organisms, classified as a monosaccharide.
Enthalpy (H)
The total chemical energy contained in a substance.
Change in enthalpy (∆H) + why does it occur?
The measurement of the difference in enthalpy between products and reactants, indicating energy absorbed or released during a chemical reaction.
occurs because bond breaking absorbs energy and bond formation releases energy, resulting in an overall enthalpy change.
Activation energy
The minimum amount of energy required for a reaction to occur
Exothermic reaction
net release of energy to the surroundings
enthalpy of the products is smaller than that of the reactants.
Endothermic reaction
net absorption of energy into the system
enthalpy of the products is greater than that of the reactants.
Thermochemical equations
Chemical equations that display both the chemical reaction and the change in enthalpy.
Heat of combustion
The heat released when 1 mole of fuel undergoes complete combustion, measured in kJ/mol or kJ/g.
Incomplete combustion
Occurs when the supply of oxygen is limited, producing water, carbon monoxide, and/or carbon.
Bond enthalpy
The average value of enthalpy per mole required to break a given bond in the gas phase, indicating the difficulty of breaking a bond.
Oxidisation
The loss of electrons, also defined as the gain of oxygen according to IUPAC.
Standard conditions (SLC)
1M
298 K (25ºC)
100 kPa
Calorimetry
An experimental technique used to determine the heat of combustion by measuring temperature changes.
Chemical reaction
A process that involves the transformation of reactants into products, accompanied by energy changes.
Calibration Factor (CF) + purpose
The energy required to increase the temperature of a set quantity of water within a calorimeter by 1ºC.
energy is needed to heat the water and the internal components of the calorimeter
Without calibration, the enthalpy value obtained will always be less than the true value for a given calorimeter using a fixed quantity of solution.
What are the limitations of HPLC?
no info on the functional groups of any compounds
retention time is not unique (some compounds have very similar retention times)
dependence on reference standards - without a calibration curve, it is difficult to assign peaks
Dissolution
The process where a solute in a gaseous, liquid, or solid phase dissolves in a solvent to form a solution.
Ways to decrease energy loss in calorimetry
Placing a lid on the beaker
insulating the beaker
ensuring sufficient oxygen is present
Solution Calorimeter
A calorimeter where the reaction takes place in solution.
Limiting Reagent
The chemical that is fully consumed in a chemical reaction and limits the amount of product that can be made.
Excess Reagent
The chemical that remains after the limiting reagent has been fully consumed, determining the quantity of products.
What is the relationship between temperature and volume?
Temperature is proportional to volume. T*V = constant
e.g. double the temperature means volume also doubles
Reduction
The gain of electrons, gain of hydrogen, or loss of oxygen.
Conjugate Redox Pair
Two chemical species where one is the product of either oxidation or reduction from the first chemical.
Galvanic Cell
A type of electrochemical cell in which chemical energy is converted into electrical energy.
Fuel Cell
A type of galvanic cell that continuously generates electricity through a spontaneous redox reaction
Electrode
The site of oxidation and reduction in an electrochemical cell.
Salt Bridge
A component that allows for the movement of ions and prevents charge build-up in half-cells.
Inert Ions
Ions that do not participate in redox reactions and do not form precipitates with common ions in solution.
Spontaneous reaction
Occurs when an oxidising agent reacts with a reducing agent, with the oxidising agent sitting higher than the reducing agent on the electrochemical series.
Standard hydrogen electrode
The half-cell against which Eº values are determined, with an Eº value arbitrarily assigned as zero.
Principles + design features of galvanic cells
Two half-cells – each contains an electrode in contact with an electrolyte solution of its own ions.
Electrodes –
Metals often act as electrodes (e.g., Zn, Cu).
If no solid conducting metal is present (e.g., for gases or ions only), an inert electrode like platinum or graphite is used.
Salt bridge (or porous separator) – completes the circuit by allowing ion migration to maintain electrical neutrality, without direct mixing of solutions.
External circuit – a wire connecting the electrodes, allowing electron flow from the anode (oxidation site) to the cathode (reduction site).
Direction of electron flow – always from the anode (negative electrode) to the cathode (positive electrode).
Porous electrodes
Have larger surface area to increase cell efficiency and rate of chemical reaction, allowing gaseous reactants to spread more evenly.
Biofuels VS Fossil fuels
Biofuels are often described as carbon neutral because the carbon dioxide released during combustion almost neutralises the carbon dioxide absorbed by plants during photosynthesis.
however extra CO2 is emitted during cultivation, processing and transport
Fossil fuels result in a net increase in the CO2 being released into the atmosphere
Advantages and disadvantages of fuel cells
advantages:
More efficient than combustion engines due to direct conversion of chemical energy into electrical energy.
quieter
bioethanol is closer to carbon neutral than fossil fuels (assuming question asks for ethanol fuel cell vs combustion engine powered by fossil fuel)
produce less greenhouse gases
disadvantages:
safety concerns associated with storage and transport of fuels
High production costs (expensive catalysts, hydrogen via electrolysis using renewables)
Most hydrogen today comes from fossil fuels (steam reforming of natural gas) → not fully “green”.
Infrastructure limitations makes large-scale adoption difficult.
Production of Hydrogen Gas
steam reforming
electrolysis of water: is a process that uses electricity to split water into hydrogen and oxygen gas. → NO CO2 PRODUCED
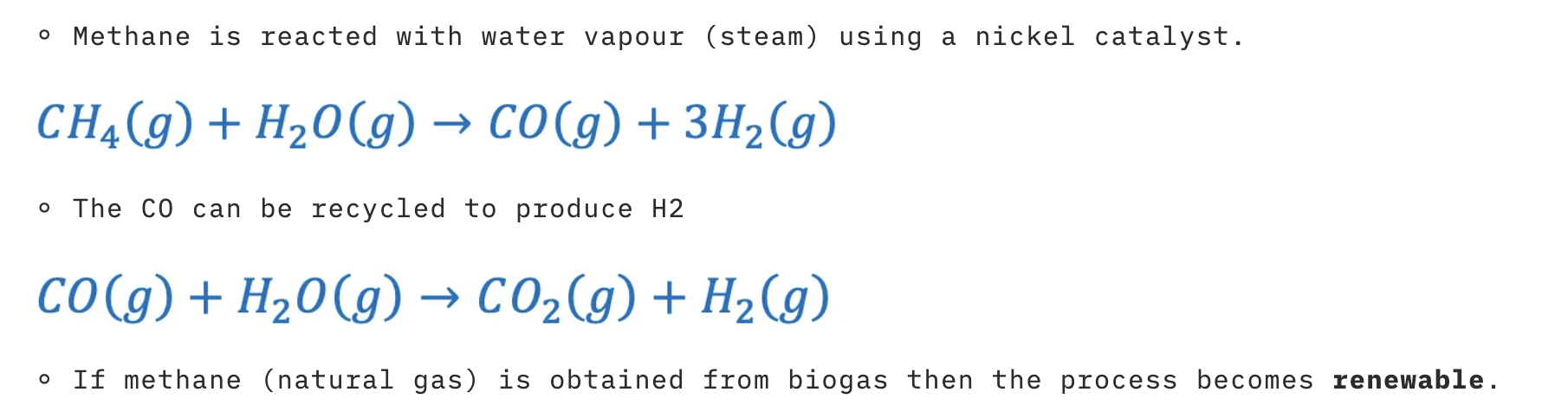
uses and limitations of electrochemical series
USES
identifies which species is more likely to be oxidised or reduced
used to deduce overall equations
determine maximum cell voltage under SLC
LIMITATIONS
Only accurate at SLC and when 1 M concentration solutions.
no information about rate of reaction is provided.
no info on extent of reaction
common design features of a fuel cell
Two electrodes
Anode: site of fuel oxidation
Cathode: site of oxidant reduction
Often porous and coated with a catalyst (like Pt) to increase ROR
Electrolyte / ion-exchange membrane
Allows only certain ions (e.g., H⁺ in PEM fuel cells, OH⁻ in alkaline fuel cells) to pass between electrodes.
Prevents fuel and oxidant mixing directly.
Porous electrodes
Provide large surface area and good diffusion for gaseous reactants.
Continuous reactant supply
External circuit
Separator / membrane layer
Keeps fuel and oxidant apart but allows ionic conduction, maintaining charge balance.
Open System
A system in which matter and energy can be exchanged with the surroundings.
Closed System
A system in which only energy is exchanged with the surroundings.
Collision Theory
there must be sufficient kinetic energy to overcome the activation energy barrier.
particles must collide with the correct orientation
example question: why don’t all reactions happen instantaneously?
Extent of Reaction
refers to how much of product that will be present, typically at equilibrium.
aka, the ratio of products to reactants.
Rate of Reaction
the change in concentration of the product/reaction per unit time.
Surface Area Effect
Increasing the surface area increases the number of particles exposed and able to collide per unit time, resulting in more successful collisions.
Concentration Effect
Having a larger concentration results in many reactant molecules available for collisions.
Temperature Effect
If the temperature increases, the proportion of reactant molecules that have sufficient energy to react successfully increases.
It also increases the amount of kinetic energy between particles, thus increasing the frequency of collisions, hence rate of chemical reaction.
Catalysts
Catalysts reduce the activation energy by providing an alternative pathway for the reaction to occur.
this allows a greater proportion of collisions to exceed the activation energy barrier, speeding up the rate of reaction.
not consumed in a reaction
allow reactions to be carried out at a lower temperature
Irreversible Reactions
Reactions where the products are not able to collide with themselves to reform the reactants, e.g., combustion reactions.
Reversible Reactions
Reactions where the products can collide with themselves to return to the reactants, e.g., redox reactions.
indicated by double arrow
Dynamic Equilibrium
Occurs in a closed system at a fixed volume where the rate of the forward reaction equals the rate of the backward reaction.
Equilibrium Law
When a chemical reaction is at equilibrium, the ratio of the concentration of products to reactants will always be a specific value at a set temperature.
DO NOT include liquid water in equilibrium expression when other reactants are aqueous solutions
Le Chatelier's Principle
States that the system will shift equilibrium to partially oppose any change made.
High Temperature Effect
Increases the reaction rate but may reduce yield of products in exothermic reactions.
Low Temperature Effect
Favorable for product yield in exothermic reactions but slows the rate of reaction significantly.
Principles and operating conditions of electrolytic cells
Electrolysis: non-spontaneous redox reaction driven by an external direct current power source.
Electrons are forced into the cathode and drawn from the anode
Products form at the electrodes and may need to be removed as they form to:
prevent spontaneous reverse reactions,
improve efficiency,
enable product collection (e.g., Cl₂ gas removal).
Common design features
Power source
Supplies sufficient voltage to overcome the reaction’s non-spontaneity and cell resistance.
Electrodes
Must conduct electricity and be chemically suited to the reactions.
Inert electrodes (e.g., graphite, platinum) when electrode material should not react.
Active electrodes (e.g., Cu in copper refining) when electrode itself participates in the reaction.
Electrolyte
Must provide mobile ions to carry charge.
May be molten (e.g., molten NaCl) or aqueous solution (e.g., NaCl(aq)).
Choice of state affects products (water may compete as a reactant in aqueous solutions).
Membranes
Physical barriers (e.g., diaphragms, membranes) can prevent products from mixing.
To facilitate the flow of ions to the cathode to complete the circuit
Anhydrous
A term describing a substance that contains no water.
Applied voltage
The voltage that must exceed the predicted value from the electrochemical series for a reaction to occur.
Molten electrolytes
Electrolytes in a liquid state used when water is a stronger oxidizing agent than the ions present.
Electroplating
The process of coating an object with a thin layer of metal to protect a more reactive layer or to retouch a worn surface.
Faraday's first law of electrolysis
States that the mass of a substance produced at an electrode is directly proportional to the total electric charge (Q) passed through the electrolyte.
Charge (Q)
Measured in coulombs (C), it represents the total electric charge passed through the electrolyte.
Faraday's second law of electrolysis
States that to produce 1 mole of a metal, a whole number of moles of electrons must be consumed.
Nature of Electrodes
Some materials have lower conductivity or reactivity. Inert electrodes (like Pt) don't participate in reactions but must still conduct electrons well.
Features and operating principles of a secondary cell
General operating principle
Discharge (galvanic)
Recharge (electrolytic)
Common design features
Electrodes
Must undergo reversible redox reactions (so products formed on discharge can be converted back on recharge).
Stable, durable, able to withstand repeated cycling.
Electrolyte
Allows for the movement of charged ions
Can be liquid, gel, or solid (but not ionic solid because ions cannot move freely)
External circuitry
Allows electrons to flow during discharge to a device.
During recharge, connects to a DC power supply that pushes current in the opposite direction.
Requirements to be able to recharge a secondary battery
The discharge reaction must be reversible
the products of the discharge reaction MUST remain in contact with the electrodes
the products of the discharge reaction MUST NOT participate in side reactions
a voltage greater than the discharge reaction must be applied during recharge
Pros & Cons & solutions of Hydrogen Fuel
Advantages:
Hydrogen has a high energy density
the only product of hydrogen combustion is water.
less GHG emissions
Problems/disadvantages
Process requires constant energy source, and renewable energy is not always used
Highly flammable and leaks easily
Hydrogen gas products take up a lot of space (not compressed) making it hard to store.
Expensive
green hydrogen via electrolysis requires a lot of energy
solution:
Stored in containers that can maintain high pressure and very low temperatures.
Produce hydrogen where it’s used (e.g. at refuelling stations) to avoid long-distance transport.
use renewable energy or obtain hydrogen from artificial photosynthesis
PEM electrolysis
Polymer electrolyte membrane.
Concept: Uses a solid polymer electrolyte (proton-exchange membrane) that allows H⁺ ions to pass but blocks gases (O₂, H₂).
Powered by renewable energy sources (like solar or wind energy)
Innovation: compact design, fast response, high purity hydrogen.
REQUIRES POROUS ELECTRODES
Advantages of innovation:
High efficiency when powered by renewables.
Modular, scalable, suitable for decentralised hydrogen production.
Produces green hydrogen with no carbon footprint.
Disadvantages:
Cost: Expensive catalysts (Pt, Ir) and membranes
Energy input: Still needs significant electricity input.
Artificial photosynthesis
Concept: Mimics natural photosynthesis using catalysts to directly harvest sunlight for water splitting.
Water oxidation catalyst at the anode generates O₂ and protons.
Proton reduction catalyst at the cathode reduces H⁺ to H₂.
uses photoelectrodes
Equations (acidic medium):
Water oxidation (anode):
2H₂O(l) → O₂(g) + 4H⁺(aq) + 4e⁻Proton reduction (cathode):
4H⁺(aq) + 4e⁻ → 2H₂(g)Overall:
2H₂O(l) → 2H₂(g) + O₂(g)
Advantages of innovation:
Directly couples sunlight to chemical fuel (no need for external solar cells).
Potential for highly sustainable, low-cost hydrogen production.
Could provide distributed hydrogen production using abundant inputs (sunlight + water).
Disadvantages:
high development cost
Catalysts and photo-absorbing materials often degrade quickly when exposed to sunlight, oxygen, and water.
low efficiency (currently)
homologous series
A family of hydrocarbons where each successive molecule differs by a -CH2- unit
Saturated
all covalent bonds between carbon atoms are single bonds
structural isomers
same chemical formula, bonded in different arrangement
Cyclohexane
a saturated cyclic alkane with the formula C6H12
addition reaction
addition of a smaller molecule to make a single product
polymer
structure made up of repeating monomers
degree of unsaturations
Refers to how many hydrogen atoms a molecule contains compared to the maximum possible number for that number of carbon atoms.
(C=C bonds)
benzene
has formula C6H6
phenyl group:
A substituent group derived from benzene by removing one hydrogen atom.
Formula C₆H₅–.
must be attached to another group, cannot exist on its own
Why do tertiary alcohols not oxidise?
The carbon bearing the –OH group is bonded to three other carbons, so it has no C–H bond available to break.
oxidation requires removal of that hydrogen
Transesterification reaction
A chemical reaction between vegetable oils/animal fats and an alcohol using a catalyst.