Topic 18: Organic Chemistry — Arenes
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
Describe the structure of benzene
an arene consisting of a ring of 6 carbon atoms with 6 hydrogen toms. The outer electron from the p-orbital of each carbon atom is delocalized into the center to form a ring making it very stable. The overlap of electrons results in the formation of π bonds.
why was Kekule model for benzene dis-proven
all C—C bonds are the same length
less reactive than alkenes
ΔH of hydrogenation is lower than expected
resistance to bromination
reaction mechanism of benzene
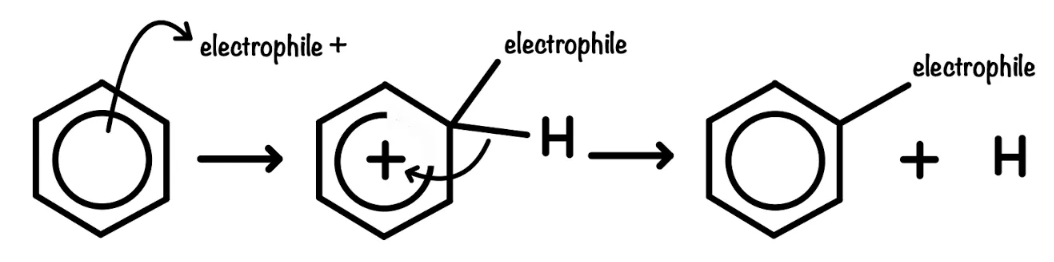
why does benzene undergo electrophilic substitution
The high electron density of the ring makes it open to attacks by electrophiles. Adding to the ring disturbs the delocalized electron system, so we substitute a hydrogen atom on the ring. Because the mechanism involves the initial disruption to the ring, electrophiles must be more powerful than those which react with alkenes.
nitration of benzene
reactant: HNO3
product: nitrobenzene + H2O
reagent: conc. nitric acid, conc. sulphuric acid catalyst
condition: reflux at 55
electrophile generation: 2H2SO4 + HNO3 ↔ 2HSO4¯ + H3O+ + NO2+
halogenation of benzene
reactant: Cl2
product: C6H5Cl + HCl
reagent: chlorine, halogen carrier catalyst
condition: reflux in presence of a halogen carrier
electrophile generation: Cl2 + FeCl3 + FeCl4¯ + Cl+
Friedel-Crafts alkylation of benzene
reactant: C2H5Cl
product: C6H5C2H5 +HCl
reagent: halogenoalkane + anhydrous AlCl3 catalyst
condition: room temp, ether solvent
electrophile generation: C2H5Cl + AlCl3 + AlCl4¯ + C2H5+
Friedel-Crafts acylation of benzene
reactant: RCOCl
product: C6H5COR + HCl
reagent: acyl chloride, anhydrous AlCl3
condition: reflux 50ºC, ether solvent
bromination of benzene
reactant: Br+
product: C6H5Br + H+
reagent: bromine, AlBr3 catalyst
condition: heat under reflux
electrophile generation: Br2 + AlBr3 + AlBr4¯ + Br+
combustion
2benzene + 15O2 → 12CO2 + 6H2O
hydrogenation
reactant: 3H2
product: C6H12
reagent: Ni catalyst
condition: heat under pressure
sulfonation of benzene
reactant: H2SO4
product: C6H5SO3H + H2O
reagent: fuming sulfuric acid
condition: warm at 40ºC
electrophile generation: fuming sulfuric acid is made by dissolving sulfur(VI)oxide in concentrated sulfuric acid
why does benzene resist bromination?
Benzene’s delocalized electron system is very stable. The electrons are distributed evenly throughout the delocalized electron system and therefore benzene is unable to polarize a non-polar molecule like bromine.
Why does phenol react with bromine water more easily than benzene?
Phenol has an alcohol group attached to the carbon rings. the lone pair of electrons on the oxygen atom is delocalized into the benzene ring, activating it. This causes an increase in the electron density and therefore induces a dipole in bromine molecules.