Electrophilic substitution
1/26
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
27 Terms
Which kinds of reactions does benzene undergo and why?
Electrophilic substitution
Benzene has a region of high electron density above and below the plane of the carbon ring, which attracts nucleophiles
The delocalisation makes the benzene very stable, and the reactions benzene undergoes aims to preserve this stability
Substitution means that they retain their delocalisation energy while addition reactions disrupt the delocalised system
When drawing mechanisms for benzene, what are the general steps?
Overall equation
Forming the electrophile
Mechanism
Reforming the catalyst
Give the general equation for all reactions of benzene
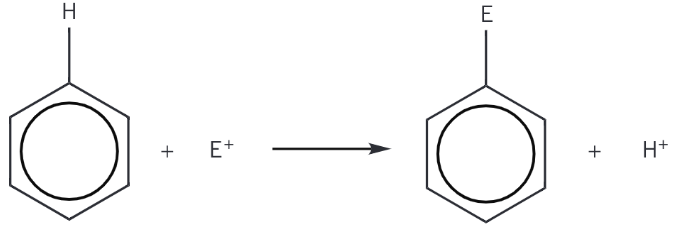
What is nitration?
When one of the hydrogen atoms on the benzene ring is replaced by a nitro, -NO2, group
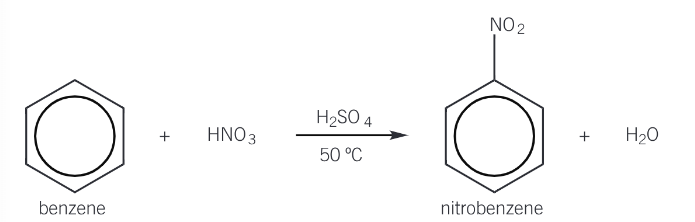
Give the conditions for nitration of benzene
Concentrated nitric acid (nitrates ring)
Concentrated sulfuric acid (catalyst)
Around 50°C
If below 55°C - monosubstituion
Above 55°C - multisubstitution
Show the overall reaction of the nitration of benzene
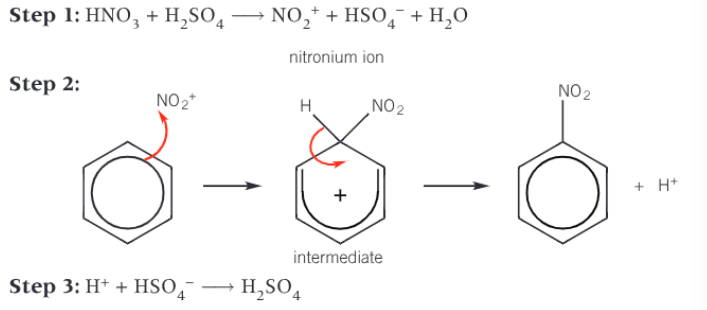
Show the whole mechanism of the nitration of benzene
Electrophile (NO2+) is produced by the reaction of concentrated nitric acid and concentrated sulfuric acid
Electrophile accepts a pair of electrons from the benzene ring to form a dative covalent bond
The organic intermediate formed is unstable and breaks down to form the organic product and a H+ ion - the benzene ring is now stable
H+ ion reacts with HSO4- ion to regenerate catalyst
What is required for the halogenation of benzene and why? Give some examples
A catalyst known as the halogen carrier
Benzene will not react with a halogen alone as it is too stable
E.g. AlBr3 / FeBr3 and AlCl3/FeCl3
Give the overall equation for the bromination of benzene
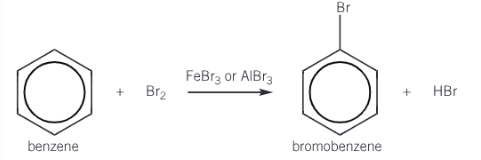
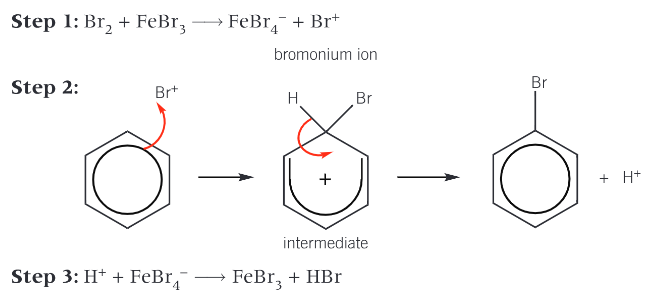
Give the overall mechanism of bromination of benzene
Electrophile (Br+) is generated when halogen carrier catalyst reacts with bromine
Bromonium ion accepts a pair of electrons from the benzene ring to form a dative covalent bond
Organic intermediate is unstable and breaks down to form the organic product (bromobenzene) and a H+ ion
H+ reacts with FeBr4- ion to regenerate FeBr3 (catalyst)
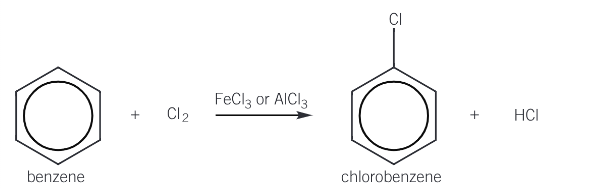
Give the overall equation for the chlorination of benzene
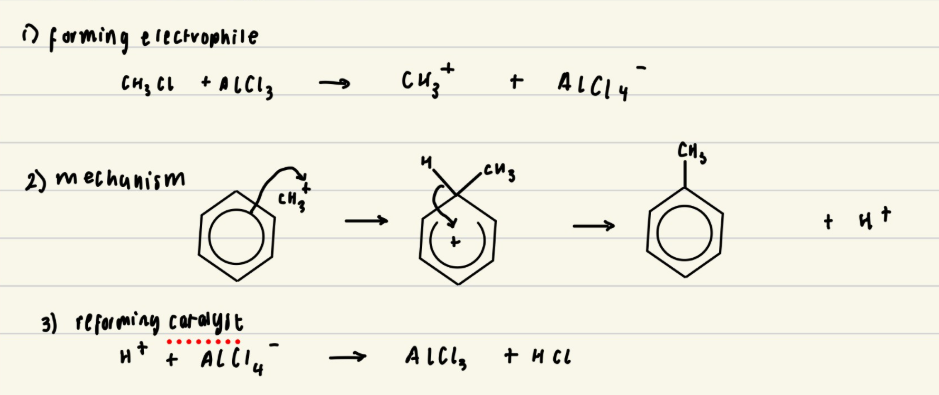
What is alkylation?
The substitution of a hydrogen atom in the benzene ring by an alkyl group (R) where benzene is reacted with a haloalkane
Give the reagents and conditions needed for alkylation
Alkyl chloride (RCl)
Halogen carrier catalyst (AlCl3) which generates the electrophile
Anhydrous conditions
How does alkylation increase the number of carbon atoms?
By forming carbon-carbon bonds
Give the equation for the alkylation of benzene with chloroethane
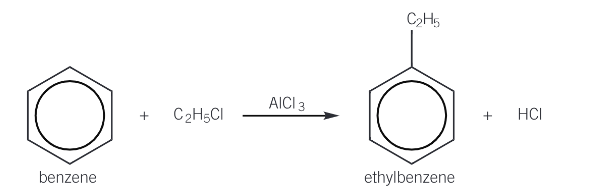
Give the mechanism for alkylation of benzene with CH3Cl
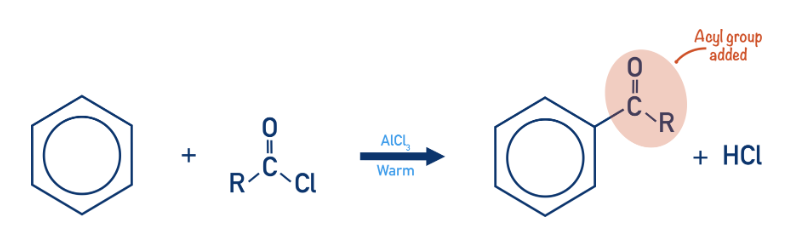
What is acylation?
When benzene reacts with an acyl chloride to form an aromatic ketone
Give the conditions and reagents for acylation
Acyl chloride (RCOCl)
Halogen carrier catalyst (AlCl3)
Anhydrous conditions
Give the overall equation for acylation of benzene
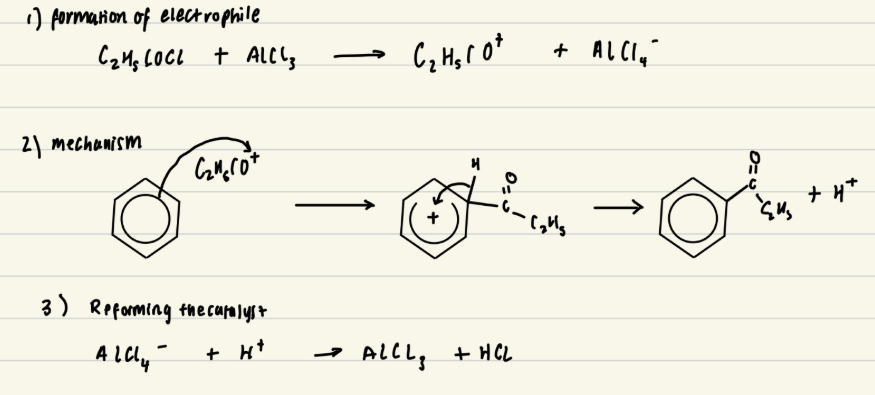
Give the mechanism for the acylation of benzene with C2H5COCl
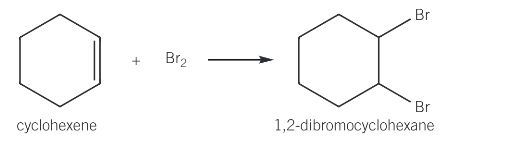
What type of reaction is the reaction of alkenes with bromine? Give the reaction of cyclohexene and bromine
Electrophilic addition
Give the mechanism for the reaction of cyclohexene and bromine
π bond in alkene contains localised electrons above and below plane of the two carbon atoms to produce an area of high electron density
Dipole is induced in non-polar bromine molecule, to make Br2 polar
Br2 is now an electrophile
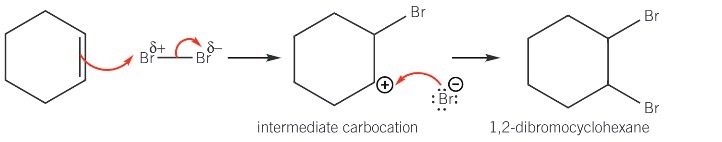
Explain why unlike alkenes, benzene does not react with bromine unless a halogen carrier is present
Benzene has delocalised π electrons above and belwo the plane of carbon atoms in a ring structure while alkenes have π electrons localised above and below carbon atoms in a double bond
The electron denisty around any two carbon atoms in a benzene ring is less than that in a C=C double bond in alkenes
Insufficient electron density around any two carbons to polarise Br2, preventing any reaction from taking place
Halogen carrier is needed to generate a more powerful electrophile (Br+)
Give the similaries between cyclohexene and benzene
Six carbon ring
Hydrocarbons
Flammable
Non-polar
Give the differences between cyclohexene and benzene

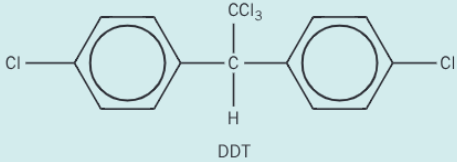
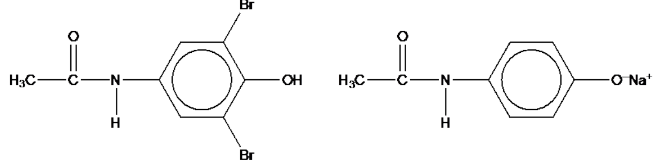
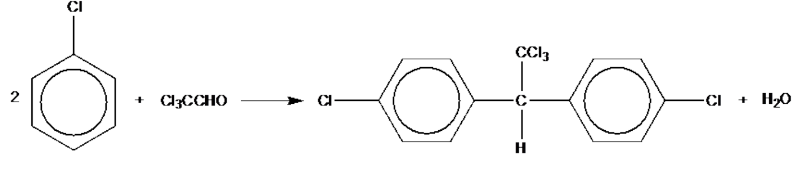
Chlorobenzene reacts with trichloroethanal, Cl3CCHO, to produce the pesticide DDT. Construct an equation for the reaction of chlorobenzene with trichlorethanal to form DDT
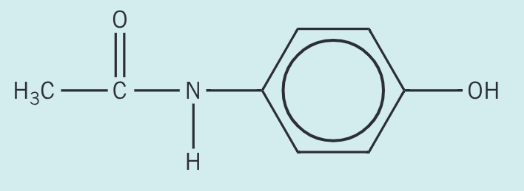
Separate samples of paracetamol are reacted with bromine, Br2, and with cold sodium hydroxide, NaOH. Draw the structures of possible organic products formed in each reaction