3.12 Properties of Photons
1/39
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
40 Terms
What happens to electrons when light shines on the surface of metal?
Ejected from the surface
The effect where when light shines on the surface of metal, electrons are ejected from the surface:
Photoelectric effect
What does the ‘photo’ in the photoelectric effect mean?
Electrons are ejected using light
The photoelectric effect is based on:
Light having wave-like and particle-like properties
What did Albert Einstein predict about light?
It has wave-like and particle-like properties
Light is composed of:
Photons
Energy of photons =
Planck’s constant times frequency
By measuring the amount of energy needed to remove the electrons we can deduce:
Binding energy
How tightly the electrons are being held in the atom
Binding energy
Greater energy-to-remove-electrons values indicate:
The electrons that are closest to the nucleus or that the nucleus is a higher charge
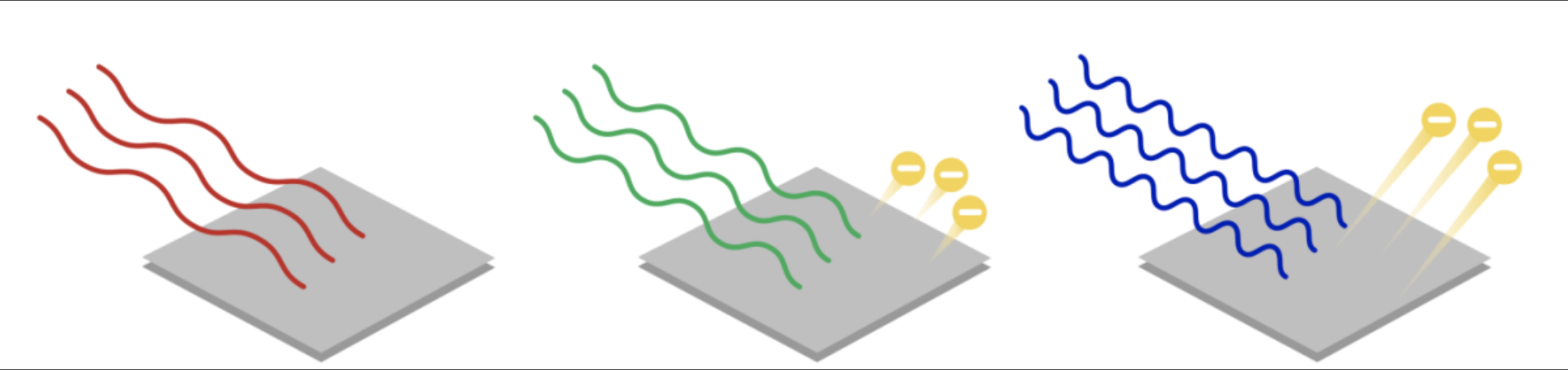
According to the image, will all light cause electrons to be ejected?
No
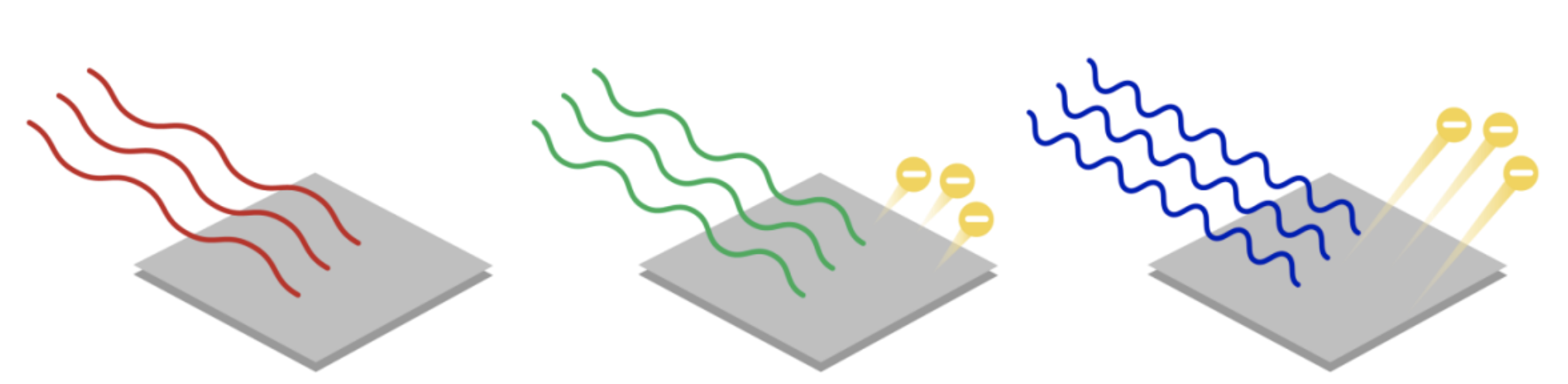
According to the image, why won’t all light cause electrons to be ejected?
Red light does not have enough energy to remove electrons
The amount of energy needed to remove an electron is measured by the:
Threshold frequency
Symbol for threshold frequency:
V0
What happens when a frequency higher than the threshold frequency is used?
The electrons will be ejected with more kinetic energy
Other than in terms of threshold frequency, the amount of energy needed to eject an electron can also be thought of in terms of the:
Threshold wavelength
The longest wavelength that will still eject an electron
Threshold wavelength
Symbol for threshold wavelength:
λMAX
Any waves that are shorter than the threshold wavelength will:
Have enough energy to eject electrons
Energy is directly/inversely proportional to frequency
Directly
Energy is directly/inversely proportional to wavelength
Inversely
Equation relating energy and frequency:
E = h ν (E = Energy (J), h = Plank’s Constant, 6.626x10^-34 Js, ν = frequency (Hz, s-1))
Equation relating frequency and wavelength:
c=νλ (c = speed of light, 3.00x10^8 m/s, ν = frequency (Hz, s-1), λ = wavelength (m))
Equation relating energy, frequency, and wavelength:
E = h c / λ (E = Energy (J), h = Plank’s Constant, 6.626x10^-34 Js, c = speed of light, 3.00x10^8 m/s, λ = wavelength (m))
Wavelengths are often measured in:
nm
Relate m to nm:
1x10^-9m = 1 nm
Waves from low to high energy:
Radio, microwaves, infrared, visible (red to violet), ultra-violet, x rays, gamma rays
The total energy of light is:
The photon
Equation involving energy of photon, kinetic energy of ejected electron, and binding energy:
Energy of photon = kinetic energy energy of ejected electron + binding energy
The energy to remove the electron is known as the:
Work function for the substance
Symbol of work function for substance:
Ф
Ф is called:
Phi
Does the work function differ for different materials?
Yes
Equation relating binding energy and threshold frequency:
Ф = hν0
Equation relating binding energy and threshold wavelength:
Ф = hc/λMAX
The energy of the photons (light) must ______ for electrons to be emitted
Meet the minimum energy
The minimum energy is given in terms of:
Being a higher frequency than the threshold frequency, ν0, or having a shorter wavelength that the λMAX
The rate of emission of the photoelectrons is known as the:
Photoelectric current
Photoelectric current is directly/inversely proportional to the intensity of the light.
Directly
If the minimum energy is met to eject electrons, the kinetic energy of the photoelectrons is dependent on:
The energy of the light used to eject the electrons