MOL100: lecture 5 protein analysis (part 2)
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
25 Terms
How many proteins does a typical human cell have?
10.000

Which 3 protein features allow purification?
-Size/mass
-Electrical charge (depends on aa composition)
-Affinity (how strong bindings)
In general how does centrifugation work? Key words
Mass/weight and gravity. Heavy things sink faster
Differential centrifugation:
1. What kind of technique?
2. How does it work?
3. Example.
1. Preparative technique, huskeregel prepare the substances for further studying.
2. Separates biological material from one another by mass/weight where heavy material sinks to bottom pellet, and lightweight material floats in supernatent.
3. DNA wrapped around histones. Differential centrifugation makes the DNA sink to bottom and histones float. Or, organelles sink bc they are heavy, and protein which are "free" in cytosol float in supernatent because they are lighter.
rate-zonal centrifugation,
1. What kind of technique?
2. how does it work?
1. Mostly analytical technique, but can also be used as preparative technique.
2. Technique to separate proteins in which a test tube containing a sucrose gradient (which establishes different levels of density in tube) has protein mixture added to it. Once centrifuged, the proteins of different densities will settle at different point in the gradient. Compared to a protein we already know the weight of.
Electrophoresis. What protein features is used, and what problems arise?
Features: mass and electrical charge. We only try to use mass. Problems arise when:
-Two proteins can have the same mass, but different shapes. Globular(round) proteins moves faster than fibrous(elongated) proteins.
-Charge: a protein with high charge will move fast, but when the same protein has a high mass it will move slow. And when another protein has a low charge it will move slow, but the same protein has a low mass which would make it fast. The two proteins are very different but would end up in the same area!
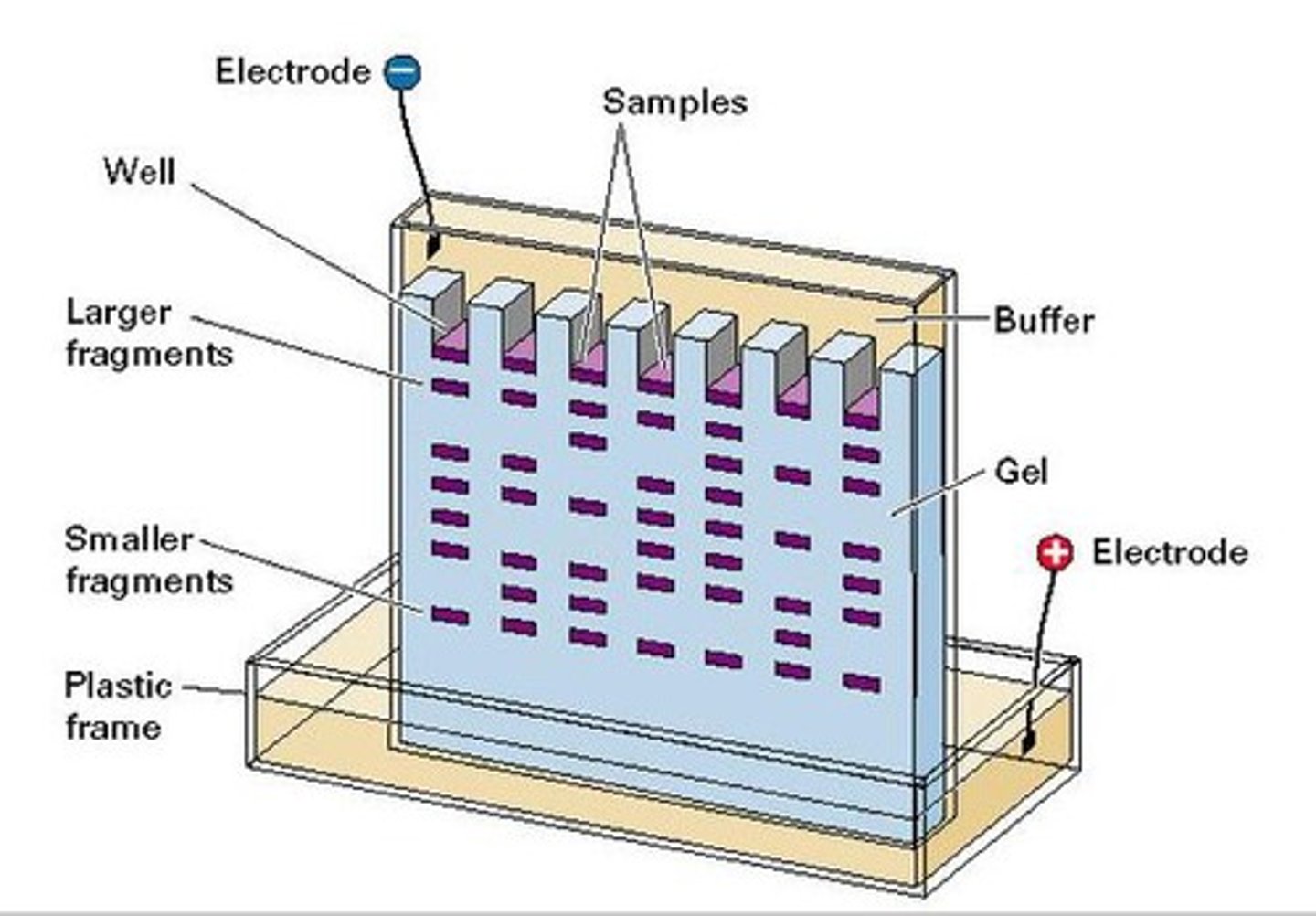
Electrophoresis. How to solve problems with mass and charge? How does this work?
SDS<3
SDS denatures protein (breaks secondary and tertiary structure) so the protein is now unfolded. Now the mass is the only thing that matters and not the shape.
SDS is very negatively charged, so strong that it masks the charge of all of the protein. The charge of the protein simply don´t matter anymore.
SDS-PAGE electrophoresis.
Finally. The proteins with low mass (the small ones) migrate faster through gel downwards to the positive bottom, while the bigger proteins moves slower in the gel on the top.
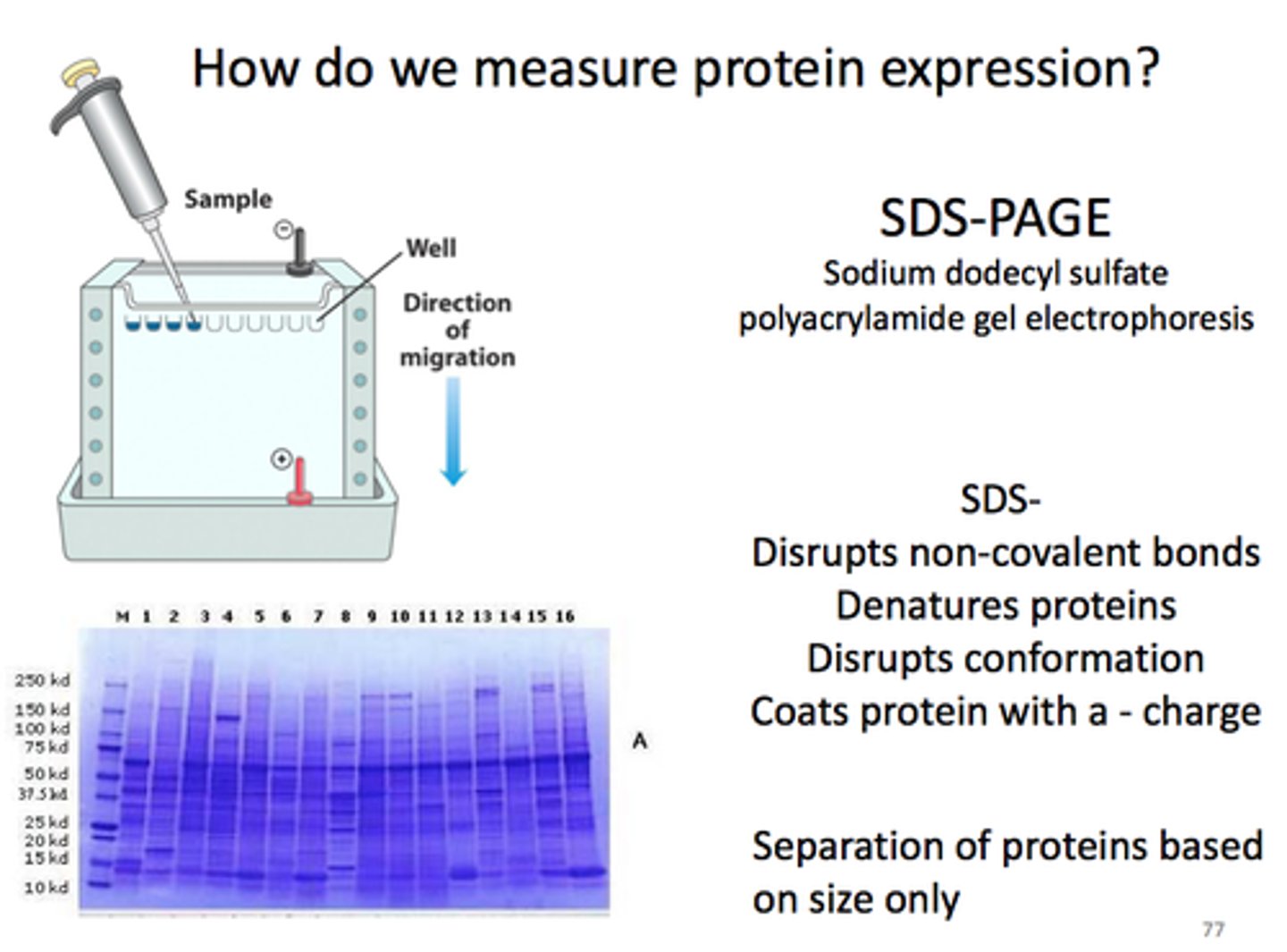
What is positive about SDS-PAGE?
It´s small and handy, often used in lab. The problems of mass, shape and charge is easily fixed by SDS.

What method is used to separate proteins in a large complex e.g. a kidney?
Liquid chromatography
Liquid chromatography, what protein features can be used?
All three of them: mass, charge and affinity
What is the method for mass chromatography?
Gel filtration chromatography
How does gel filtration chromatography work?
separate proteins based on size. Big proteins go fastest because the beads used in gel filtration have holes to trap small proteins
What is the method for chromatography based on charge?
Ion-exchange chromatography
How does ion exchange chromatography work?
Separates protein based on their change. Positive OR negative beads. Positive beads interact/"traps" negative proteins, and the positive proteins are free to migrate to the bottom. And the other way around.
How is negative proteins released from positive beads?
How does this help us separate proteins?
With salt Na+Cl-
The negative Cl- competes with the negative proteins, Cl- wants to take it´s place. Low salt concentration only disrupts weak bindings. The stronger salt concentration the more Cl- to compete the more protein is outcompeted and then becomes freeee<3
Proteins is separated according to how strong they where bound, which we already know by the amount of salt was needed to free the different proteins.
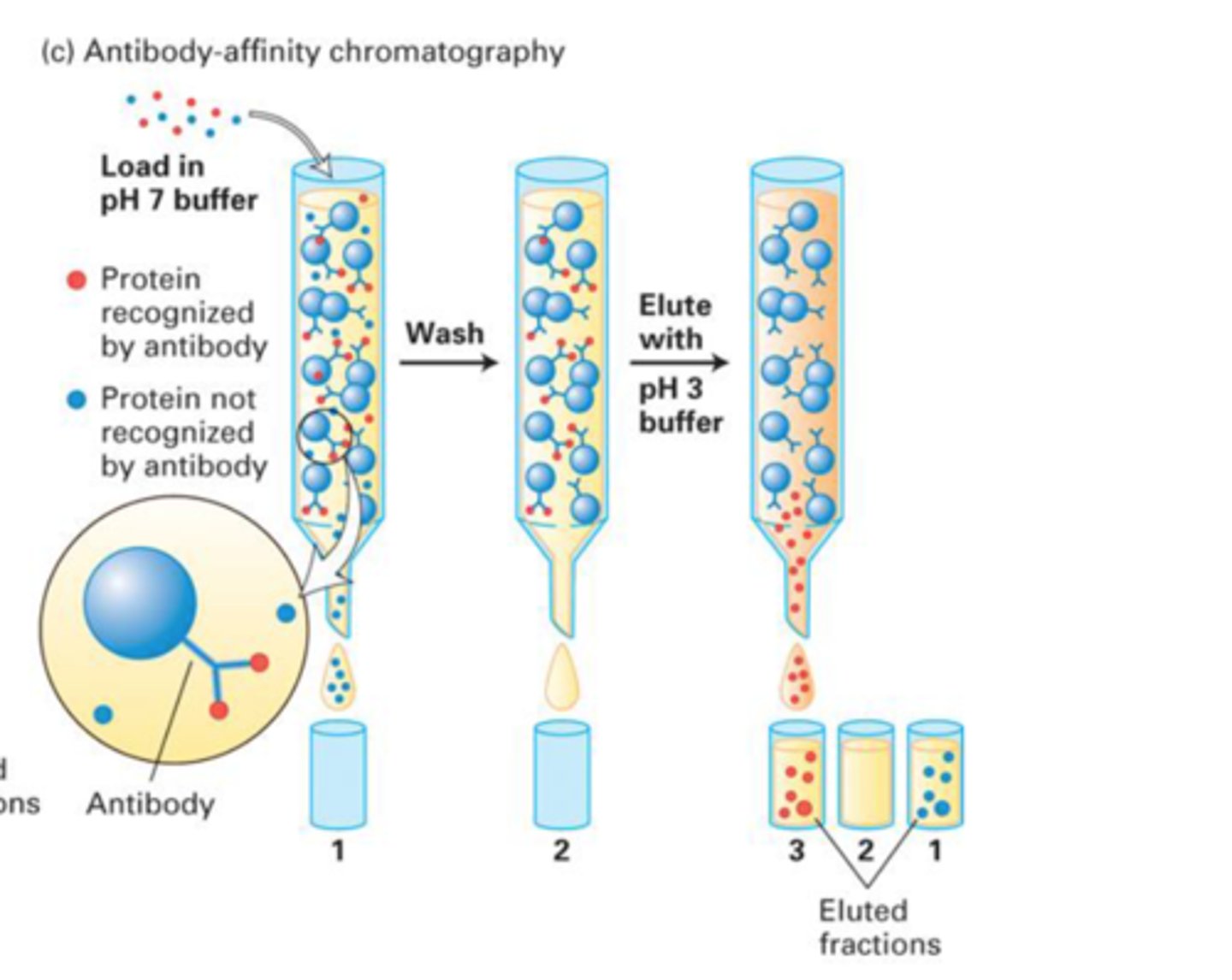
What type of chromatography uses affinity?
Affinity chromatography. Uses antibodies.
How is antibodies used in affinity chromatography?
Antibodies has a hypervariable region which only binds to one specific protein of interest. Everything else migrates to bottom and can be taken out. Now what is left in column is antibodies with protein attached. To free the proteins from antibodies we use an acidic buffer.
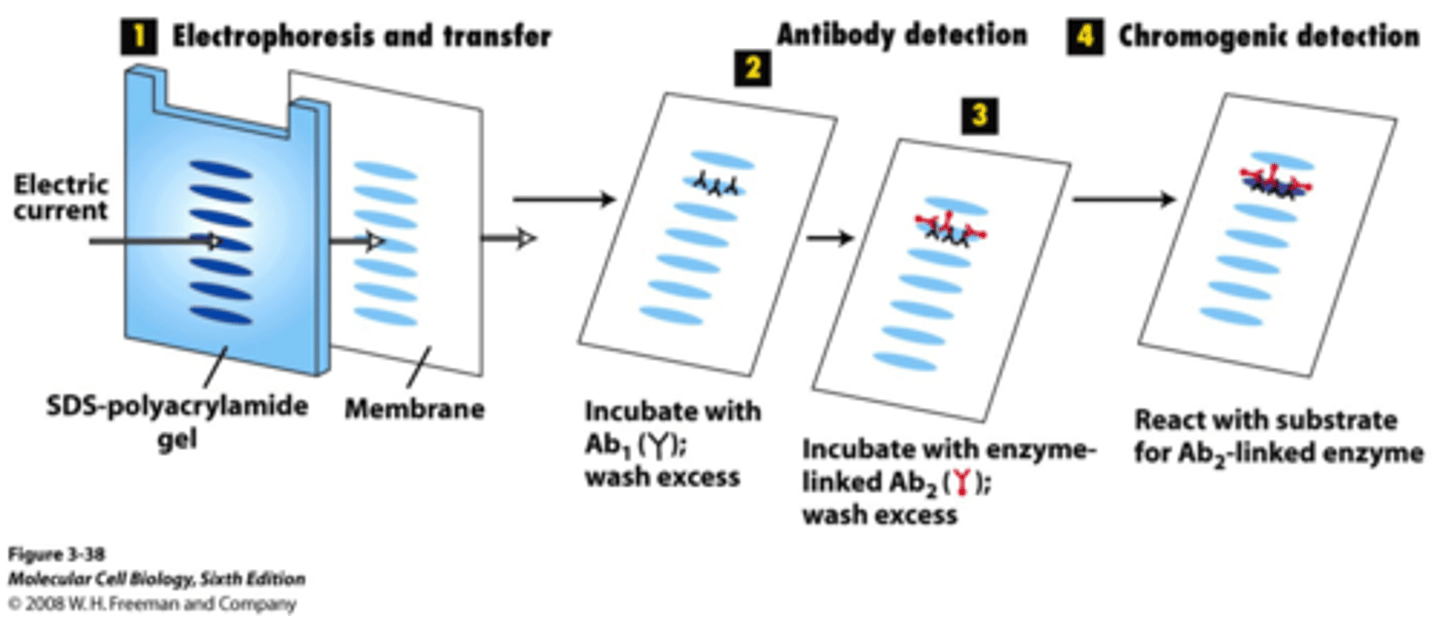
Name the two ways for detection of protein.
Immunoblotting/Western blot
Mass spectrometry
How does immunoblotting/western blot work?
What is determined?
After SDS-PAGE we know the size (bc it´s compared to a referance), but to study further we need to get the protein out of the gel. Use electric current to transfer proteins from gel on to e membrane with high affinity to all proteins. Then specific antibodies is added to detect the protein we expect to find.
Determines the protein size and abundance (mengde)
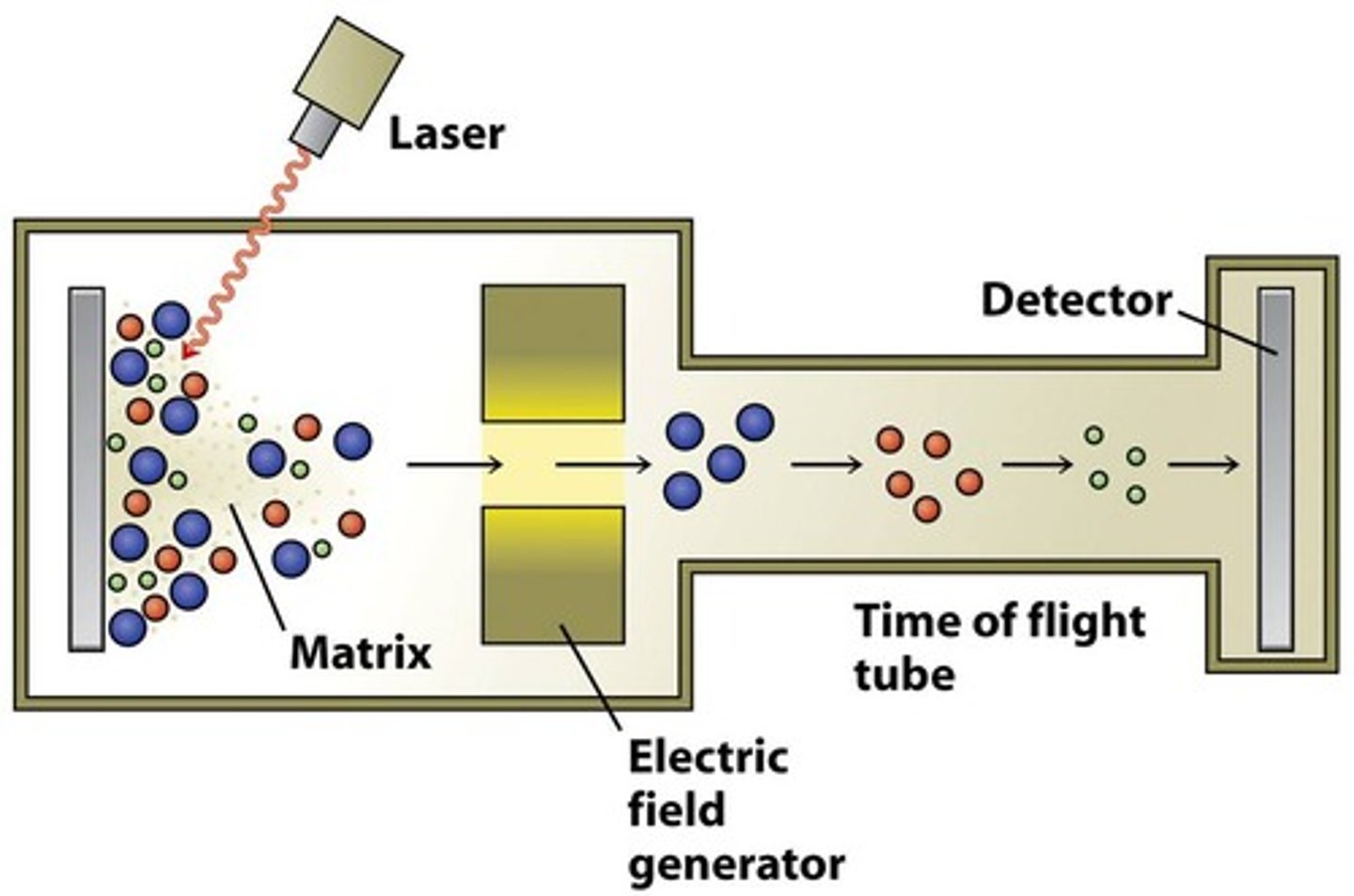
Mass spectrometry
What is analysed?
How does it work? Key words
The mass. The primary structure. Low mass is faster, higher mass is slower to hit the detector. Compared with database of the particular organism of all possible proteins encoded in genome.
Ionized. Detector. Mass. Database.
Name the two methods to analyse protein conformation?
1. X-ray crystallography
2. Nuclear magnetic resonance spectroscopy
What is challenging in x-ray crystallography?
To generate a protein crystal.
What is proteomics?
the study of the full protein set.
Proteomics detects all proteins that are present in a sample. Such a sample can be from an organ, or from one organelle, or even from a whole organism. The term proteomics is used to separate it from experiments in which only one specific protein is detected, for example by immunoblotting.
Mass spectrometry is a method for proteomics.
In mass spectrometry, all proteins in a sample are analyzed. They are cut into smaller fragments, the fragments are ionized and separated in a mass analyzer. They then hit a detector which allows to calculate the mass of the fragment. The mass is compared to the mass of all fragments that can possibly be found, and this identifies the fragment as part of a specific protein. Mass spectrometry detects millions of such fragments from a sample.