module 4
1/118
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
119 Terms
ozone layer being formed and reformed equations

CFCs depleting the ozone layer
The C-Cl bond breaks because it has the lowest bond enthalpy once the UV radiation provides sufficient energy
the chlorine radical formed is a very reactive intermediate. It can react with an ozone molecule, breaking down the ozone into oxygen
propagation step 2 regenerates a chlorine radical, which can attack and remove another molecule of ozone. It has been estimated that a single CFC molecule can promote the breakdown of 100,000 molecules of ozone

nitrogen radicals depleting the ozone layer
nitrogen oxide radicals are formed naturally during lightening strikes and as a result of aircraft travel in the stratosphere

where were CFCs used
refrigerants
air-conditioning units
aerosol propellants
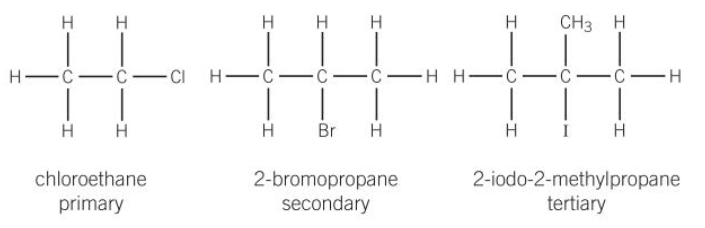
naming haloalkanes
prefix is added to the name of the longest chain to indicate the identity of the halogen
when two or more halogens are present they are listed in alphabetical order
they can be classed as primary, secondary and tertiary
reactivity of the haloalkanes
halogen atoms are more electronegative that carbon atoms
the electron pair in the carbon-halogen bond is therefore closer to the halogen atom than the carbon atom. The carbon halogen bond is polar
the carbon atom has a slightly positive charge and can attract species containing a lone pair of electrons. Species that donate a lone pair of electrons are known as nucleophiles
nulceophile
an atom or group of atoms that is attracted to an electron deficient carbon atom, where it donated a pair of electrons to form a new covalent bond.
nucleophiles include:
-hydroxide ions
-water molecules
-ammonia molecules
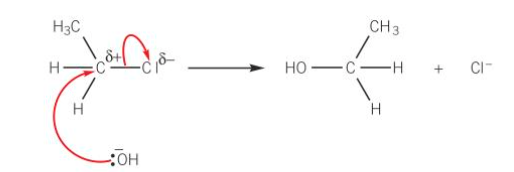
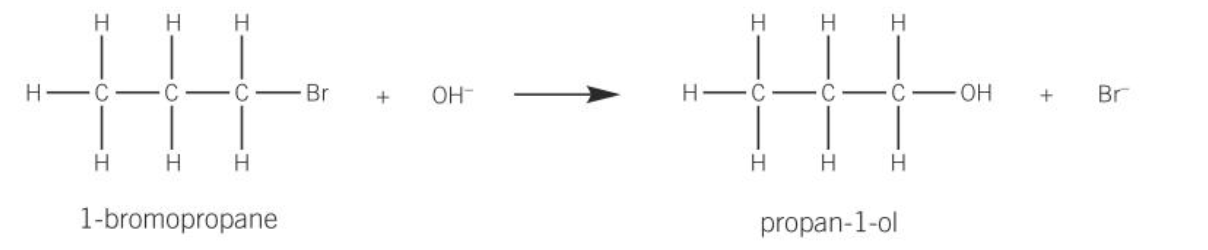
hydrolysis of halogen in nucleophilic substitution reaction
the nucleophile OH-, approaches the carbon atom attached to the halogen on the opposite side of the molecule from the halogen atom
this direction of attack by the OH- ion minimises repulsion between the nucleophile and the halogen atom
a lone pair of electrons on the hydroxide ion is attracted and donated to the carbon atom
a new bond is formed between the oxygen atom of the hydroxide ion and the carbon atom
the carbon halogen bond breaks by heterolytic fission
the new organic product is an alcohol. A halide ion is also formed
halogen atoms can be converted to alcohols using aqueous sodium hydroxide
carbon-halogen bond strength
C-F bond is the strongest carbon-halogen bond and the C-I bond is the weakest
iodoalkanes react faster than bromoalkanes
bromoalkanes react faster than chloroalkanes
fluoroalkanes are unreactive as a large quantity of energy is required to break the C-F bond
combustion of alcohols
alcohols burn completely in a plentiful supply of oxygen to produce carbon dioxide and water. The reaction is exothermic and as the number of carbon atoms in the alcohol chain increases, the quantity of heat released per mole increases
oxidation of alcohols
primary and secondary alcohols can be oxidised by an oxidising agent
the usual oxidising mixture is a solution of potassium dichromate acidified with dilute sulphuric acid
if the alcohol is oxidised then the orange solution containing dichromate ions is reduced to a green solution containing chromium ionsoxidatio
oxidation of primary alcohols
primary alcohols can be oxidised to either aldehydes or carboxylic acids. The production of the oxidisation depends on the reaction conditions used because aldehydes are themselves also oxidised to carboxylic acids
preparation of aldehydes
on gentle heating of primary alcohols with acidified potassium dichromate, an aldehyde is formed
the ensure that the aldehyde is prepared rather than the carboxylic acid, the aldehyde is distilled out of the reaction mixture as it forms
this prevents any further reactions to the oxidising agent
the dichromate ions change colour from orange to green
preparation of carboxylic acids
if a primary alcohol is heated strongly under reflex, with an excess of acidified potassium dichromate, a carboxylic acid is formed
use an excess of acidified potassium dichromate to ensure that all of the alcohol is oxidised
heating under reflex ensures that any aldehyde formed initially in the reaction also undergoes oxidation to the carboxylic acid
oxidation of secondary alcohols
secondary alcohols are oxidised to ketones. It is not possible to further oxide ketones using acidified dichromate ions
to ensure the reaction goes to completion, the secondary alcohol is heated under reflux with the oxidising mixture
the dichromate ions change colour from orange to green
oxidation of tertiary alcohols
tertiary alcohols do not undergo oxidation reactions, The acidified potassium dichromate remains orange when added to a tertiary alcohol
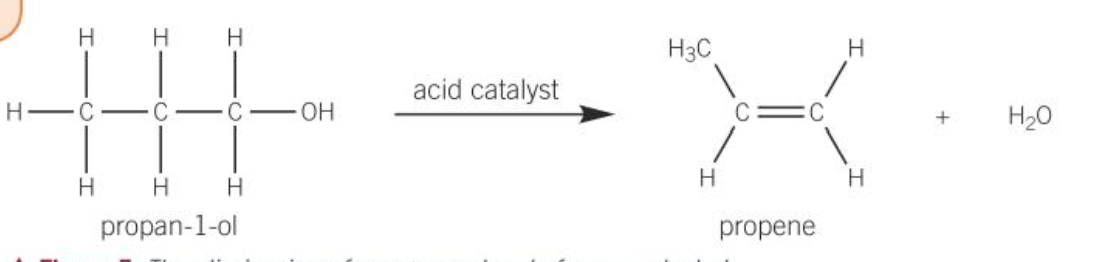
dehydration of alcohols
an alcohol is heated under reflux in the presence of an acid catalyst such as concentrated sulphuric acid or phosphoric acid
the product of the reaction is an alkene
dehydration of an alcohol is an example of an elimination reaction
dehydration reaction
any reaction is which is water molecule is removed form the starting material
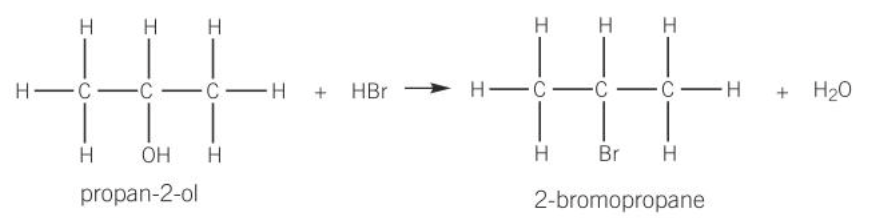
substitution reaction of alcohols
when preparing a haloalkane, the alcohol is heated under reflux with sulphuric acid and a sodium halide the hydrogen bromide is formed in site
NaBr(S) + H2SO4(aq) → NaHSO4(aq) + HBr(aq)
the HBr formed reacts with the alcohol to produce the haloalkane
naming alcohols
The suffix -ol is added to the stem containing the largest carbon chain. The position of the functional group along the chain is indicated using numbers
physical properties of alcohols
compared to alkanes they are:
less volatile
have higher melting points
greater water solubility
differences become smaller as the length of the carbon chain increases
reasons of the physical properties of alcohols
The alkanes have non-polar bonds because the electronegativity of hydrogen and carbon are very similar
the alkane molecules are therefore non-polar
the inter molecular forces between non-polar molecules are very weak London forces
alcohols have a polar O-H bond because of the different in electronegativity of the oxygen and hydrogen atoms
alcohol molecules are therefore polar
the intermolecular forces will be very weak London forces but there will also be much stronger hydrogen bonds between the polar O-H group
why is there a difference in volatility of boiling points between alkanes and alcohols
in the liquid state, intermolecular hydrogen bonds hold the alcohol molecules together
These bonds must be broken in order to change the liquid alcohol into a gas
this requires more energy than overcoming the weaker London forces in alkanes, so alcohols have a lower volatility that the alkanes with the same number of carbons
why is there a difference in the solubility of water between alkanes and alcohols
a compound that can form hydrogen bonds with more is far more water soluble that a compound that cannot
alkanes are non-polar compounds and can not form hydrogen bonds with water
alcohols such as methanol and ethanol are completely soluble in water, as hydrogen bonds form between the polar -OH group of the alcohol and the water molecules
as the hydrocarbon chain length increases in size, the influence of the -OH group becomes relatively smaller, and the solubility of longer-chain alcohols becomes more like that of hydrocarbons - solubility decreases.
classifying alcohols
alcohols can be classed as primary, secondary or tertiary. This classification depends on the number of hydrogen atoms and alkyl groups attached to the carbon atom that contains the alcohol functional group
primary alcohols
in primary alcohols, the -OH group is attached to a carbon atom which is attached to two hydrogen atoms and 1 alkyl group. (methanol is the exception and its still classed as a primary alcohol)
secondary alcohols
the -OH group is attached to a carbon atom that is attached to one hydrogen atom and two alkyl groups. Propan-2-ol and pentan-3-ol are both examples of secondary alcohols
tertiary alcohols
the -OH group is attached to a carbon atom that is attached to no hydrogen atoms and three alkyl groups. 2-methylpropan-2-ol is an example
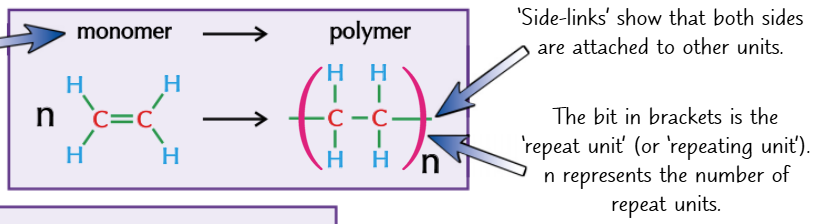
what type of polymerisation do alkenes do
addition polymerisation
made up of small alkenes called monomers
why are polymers very useful
They are very unreactive but this makes getting rid of them an issue
different solutions for getting rid of plastics
buried
reused
burned
creating biodegradable plastics
waste plastics being buried
landfill is an option for dealing with plastics. It is used when:
its difficult to separate from other waste
not sufficient in quantities to make separation financially worthwhile
too difficult to technically recycle
but because the amount of waste we generate is becoming more and more of problem, there’s a need to reduce landfill as much as possible
waste plastics can be reused
many plastics are made from non-renewable oil-fractions, so it makes sense to reuse plastics as much as possible
some plastics can be recycles by melting and remoulding them
some plastics can be cracked into monomers, and these can be used as an organic feedstock to make more plastics or other chemicals
burning waste plastics
if recycling isn’t possible for whatever reason, waste plastics can be burned - and the heat can be used to generate electricity
This process needs to be carefully controlled to reduce toxic gases for examples, polymers that contain chlorine such as PVP produce HCL when they are burnt which needs to be removed
waste gases from the combustion are passed through scrubbers which can neutralise gases such as HCl by allowing them to react with a base
biodegradable polymers
biodegradable polymers decompose quickly in certain conditions because organisms can digest them
biodegradable polymers can be made from renewable raw materials such as starch or oil fractions, such as the hydrocarbon isoprene, but at the moment they are more expensive than non biodegradable equivalents
They need certain conditions such as moisture and oxygen to biodegrade
This means that all of the polymers need to be collected and separated from the non-biodegradable plastics
There are various potential uses - eg. plastic sheeting used to protect plants from the frost can be made from polyethene with starch grains embedded in it. In the time the starch is broken down by microorganisms and the remaining polyethene crumbles into dust
scientists have also started developing photodegradable polymers which degrade when exposed to sunlight
electrophilic addition
alkene double bond opens up an atoms are added to the carbon atoms
Electrophilic addition reactions happen because the double bond has got plenty of electrons and is easily attacked by eletrophiles
electrophiles
electron pair acceptors
usually positively charged ions
polar molecules where the partially positive area is attracted to the electrons
Producing ethane from ethene
Reacts with hydrogen gas in an addition reaction to produce ethane
It will need a nickel catalyst and a temperature of 150
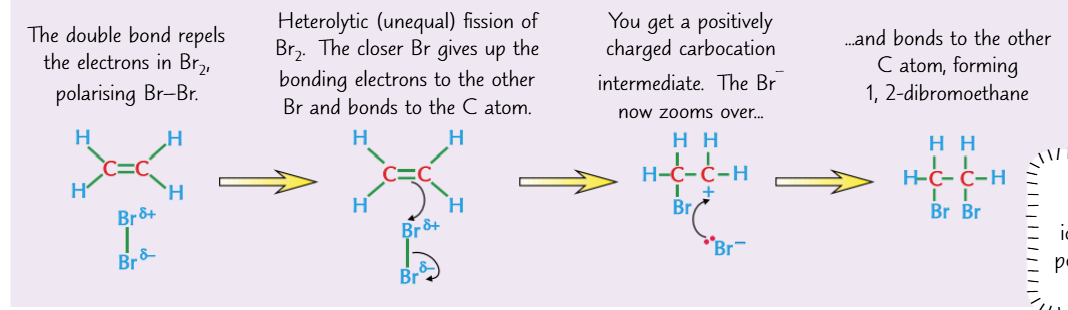
mechanism for halogens reacting with alkenes
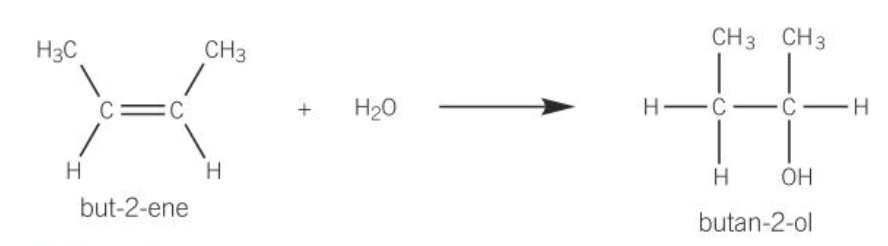
making alcohols from alkenes
made by steam hydration
300 degrees and a pressure of 60-70atm
phosphoric (V) acid catalyst is needed
Reaction is reversable
only produces a low yield of about 5% but you can recycle the unreacted alkene gas making the overall yield much better
alkene reaction with hydrogen halides
undergo addition reactions to form haloalkenes
two products formed by adding halides to unsymmetrical alkenes
carbocations with more alkyl groups are more stable because the aklyl groups feed electrons towards the positive charge
The more stable cation is more likely to form
least stable → most stable
primary → secondary → tertiary
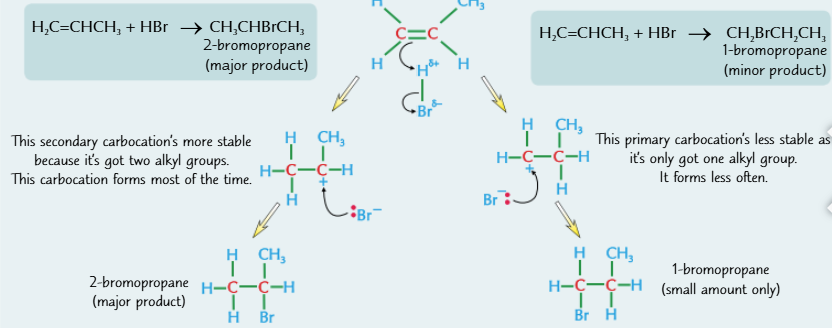
Markownikoff’s rule
The major product from addition of a hydrogen halide to an unsymmetrical alkene is the one where hydrogen adds to the carbon with the most hydrogens already attached.
Why can double bonds not rotate
Carbon atoms in a carbon carbon double bond and the atoms bonded to these carbons all lie in the same plane (they are planar)
because of the way they are arranged, They are said to be trigonal planar
The bond angles in the planar units are all 120 degrees
atoms can’t rotate around them like they can around a single bond because of the pi bonds
Atoms can still rotate around the single bonds in an alkene
The restricted rotation causes alkenes to form stereoisomers
what are stereoisomers
They have the same structural formula but a different arrangement in space
Why can alkenes form stereoisomers
Because of the lack of rotation around the double bond when the two double bonded carbon atom have two different groups attached to them then stereoisomerism can take place
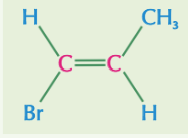
Z isomer vs E isomer of but-2-ene
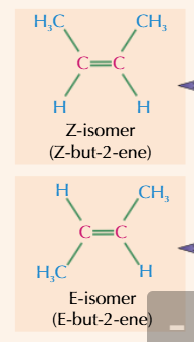
Cahn-Ingold-prelog rules to work out E vs Z isomers
atom with the highest atomic number has the highest priority
Highest priority on the same side of the double bond = Z
opposite sides = E
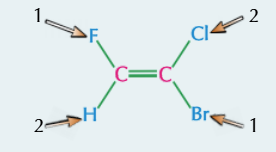
cis-trans isomers
if the carbon atoms have at least one group in common, then you can call the isomers cis or trans
‘cis’ means the same groups are on the same side of the double bond
‘trans’ means the same groups are on different sides of the double bond
unsaturated hydrocarbons
contain at least one carbon carbon double bond
general formula of alkenes
CnH2n
sigma bond
formed when two S orbitals overlap
The two S orbitals overlap in a straight line - this gives the highest possible electron density between the nucleic (single covalent bond)
The high electron density between the nuclei means there is a strong electrostatic attraction between the nuclei and the shared pair of electrons
They have a high bond enthalpy - strongest type of covalent bonds
Pi bond
The sideways overlap of two adjacent P orbitals
Its got two parts to it - one above and one below the molecular axis. This is because the P orbitals which overlap are dumb-bell shaped
Pi bonds are much weaker than sigma bonds because the electron density is spread out above or below the nuclei. This means that the electrostatic attraction between the nuclei and the shared pair of electrons is weaker, so pi bonds have a relatively low bond enthalpy
Why are alkenes more reactive than alkanes
alkanes only contains C-C and C-H sigma bonds which have a high bond enthalpy and so are difficult to break. They are also non polar so don’t attract nucleophiles or electrophiles
Alkenes are more reactive than alkanes because the double bond contains both a sigma and a pi bond
the double bond contains 4 electrons so it has a high electron density and the pi bond sticks out above and below the rest of the molecule. These two factors mean that the pi bond is likely to be attacked by electrophiles. The low bond enthalpy of the pi bind also contributes to the reactivity of alkanes
reactivity of alkanes
alkanes do not react with most common reagents
the sigma bonds are stronger
and c-c bonds are non polar
the c and H have very little differences in electronegativity
complete combustion of alkanes
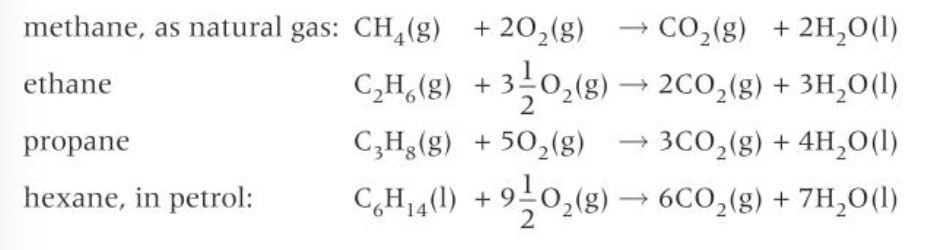
incomplete combustion of alkanes
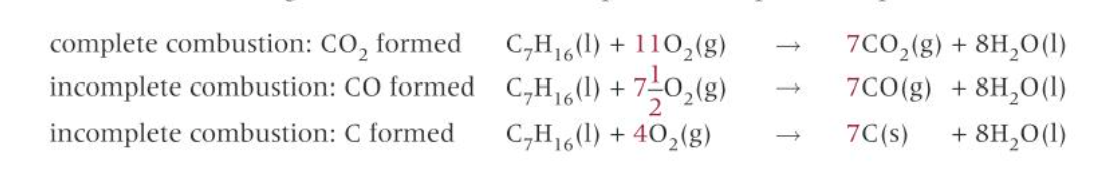
carbon monoxide
a colourless, odourless, highly toxic gas
combines with the haemoglobin which prevents them from carrying oxygen around in the bloodstream
reaction of alkanes with halogens
react in the presence of UV light
a substitution reaction
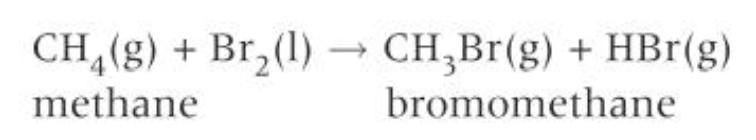
3 stages for the mechanism for the bromination of alkanes
initiation, propagation, termination
What is the mechanism for the bromination of methane an example of
radical substitution
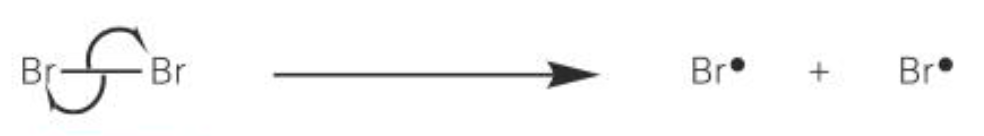
initiation
the covalent bond in a bromine molecule is broken by homolytic fission
each bromine atom takes one electron from the pair, forming two highly reactive bromine radicals
the energy for this bond fission is provided by UV ration
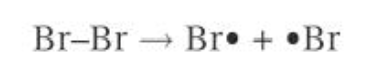
Propagation
the reaction propagates through two propagation stages, a chain reaction
In the first propagation step, a bromine molecule reacts with a C-H bond in the methane, forming a methyl radical and a molecule of hydrogen bromine
in the second propagation stage, each methyl radical reacts with another bromine molecule, forming the organic product bromomethane and another bromine radical
The new bromine radical then starts this process over again
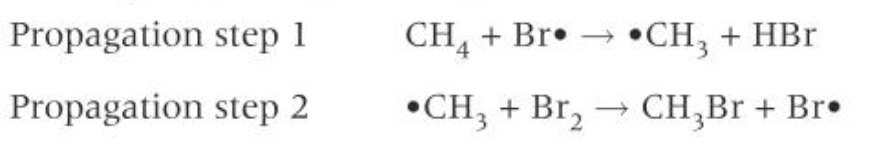
termination
two radicals collide, forming a molecule with all electrons paired
there are a number of possible termination steps
when two radicals collide and react, both radicals are removed from the reaction mixture stopping the reaction
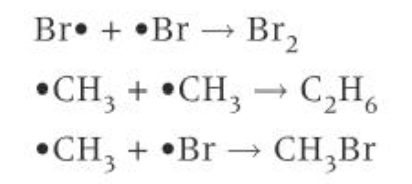
limits of radical substitution in organic synthesis
further substitution and substitution at different positions along a carbon chain
further substitution
another bromine bromine radical can collide with a bromoethane molecule, substituting a further hydrogen atom to form dibromoethane CH2Br2
further substitution can continue until all hydrogen atoms have been substituted
substitution at different points along the carbon chain
for methane and ethane, there is only one monosubstituted product possible
if the carbon chain is longer, there a more positions in the carbon chain
with further substitution, there are even more possibilities
bonding in alkanes
saturated hydrocarbons
contains only carbon and hydrogen atoms
each carbon atom is joined to 4 other atoms by single covalent bonds. These are a type of covalent bond called a sigma bond
sigma bond
a sigma bond is the result of the overlap of two orbitals, one from each bonding atom
each overlapping orbital contains one electron, so the sigma bond has two electrons which are shared between the bonding atoms
Shape of alkanes
each carbon atoms is surrounded by four electron pairs in four sigma p=bonds
repulsion between these electron pairs results in a 3D tetrahedral arrangement around each carbon atom with the bond angle being approx 109.5
the sigma bonds act as axis at which the atoms can rotate around freely
variation in boiling point of the alkanes
boiling point increases as the carbon chain increases
there are more electrons therefore the London forces get stronger
effect of chain length on boiling point
London forces act between molecules that are in close surface contact
as the chain length increases, the molecules have a large area so more surface contact is possible between molecules
the London forces in between the molecules will be greater so more energy needed to overcome the forces
effect of branching on boiling point
decreases as branching increases
fewer points of contact therefore fewer London forces
the branches get in the way and prevent molecules from getting as close to each other
types of bond fission
homolytic or heterolytic fission
homolytic fission
each of the bonded atoms takes one of the shared pair of electrons from the bond
each atom now has a single unpaired electron
each atom or groups of atoms with an unpaired electron is called a radical
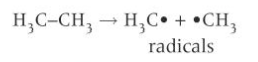
heterolytic fission
when a covalent bond breaks by heterolytic fission, one of the bonded atoms takes both of the electrons from the bond
the atom that takes both electrons becomes a negative ion
the atom that does not take the electrons becomes a positive ion
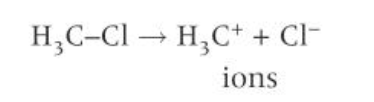
curly arrows
in a reaction mechanism, curly arrows are used to show the movement of electron pairs when bonds are being broken or made
curly arrows and homolytic fission
addition reactions
in an addition reaction, two reactants come together to from one product
substitution reaction
In a substitution reaction, an atom or group of atoms is replaced by a different atom or a group of atoms
elimination reaction
an elimination reaction involves the removal of a small molecule from a larger one. In an elimination reaction, one reactant molecule forms two larger products
structural isomerism
compounds with the same molecular formula but different functional group
isomers with the same functional group
In compounds with the same functional group, the functional group can be at different positions along the carbon chain
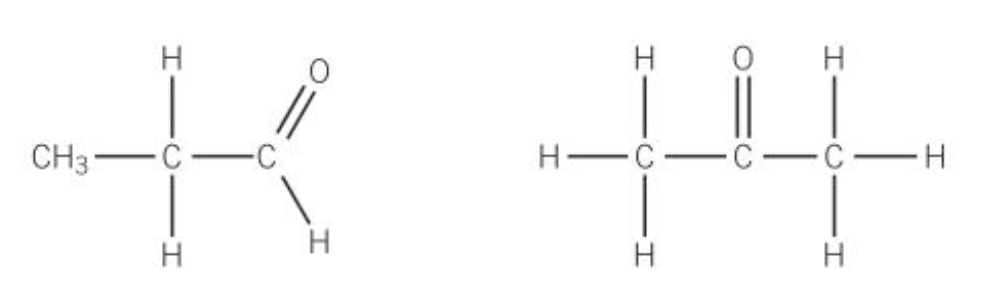
isomers with different functional groups
sometimes two molecules with the same molecular formula can have different functional groups
Eg, aldehydes and ketones with the same number of carbon atoms have the same molecular formula
detecting isomerism by smell

molecular formula
the number of type of atoms of each element present in a compound
empirical formula
the simplest whole number ratio of the atoms of each element present in a compound
general formula
the simplest algebraic formula for any member of a homologous series
displayed formula
Shows the relative positioning of all of the atoms in a molecule and the bonds between them
structural formula
uses the smallest amount of detail necessary to show the arrangement of atoms in the molecule. Branched chains are in brackets
Skeletal formula
A simplified organic formula where:
all of the carbon and hydrogen labels are removed
all bonds to hydrogen atoms are removed
a line represents a single bond
an intersection of two lines represents a carbon atom
the end of a line represents a CH3 group
aliphatic
carbon atoms are joined up to each other in unbranched or branched chains or in non-aromatic rings
alicyclic
carbon atoms which are joined to each other in ring structures with or without branches
aromatic
some or all of the carbon atoms are found in a benzene ring
alkanes
containing single carbon carbon bonds
alkenes
containing at least one double carbon carbon bond
alkynes
contains at least one triple carbon carbon bond
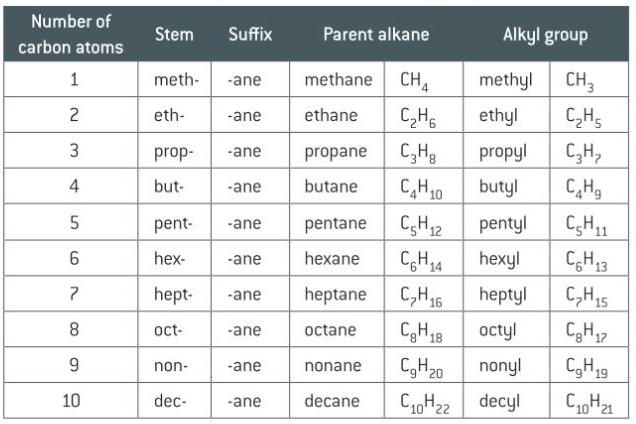
names of alkanes 1-10 carbons
How to name alicyclic alkanes
identify the longest continuous chain of carbon atoms
add the prefix cyclo
how to name alkenes
identify the longest continuous chain of carbon atoms
identify where the double bond is
combine the suffix, stem and position of where the double bond is