Chapter 25- Amino Acid Synthesis
1/4
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
5 Terms
How do amino acids obtain their nitrogen?
Primarily from glutamine or glutamate and the carbon skeletons come from intermediates of glycolysis, citric acid or pentose phosphate pathway
Which carbon skeleton gives rise to which amino acids
importance of tetrahydrofolate as a cofactor in the amino acid biosynthesis
Tetrahydrofolate carries activated one-carbon units at several oxidation levels
It is composed of 3 groups:
A substituted pteridine ring
P-aminobenzoate
A chain of one or more glutamate residues
Mammals obtain tetrahydrofolate from their diets or from microorganisms in their intestinal tracts
One-carbon group carried by tetrahydrofolate is bonded to its N-5 or N-10 nitrogen atom
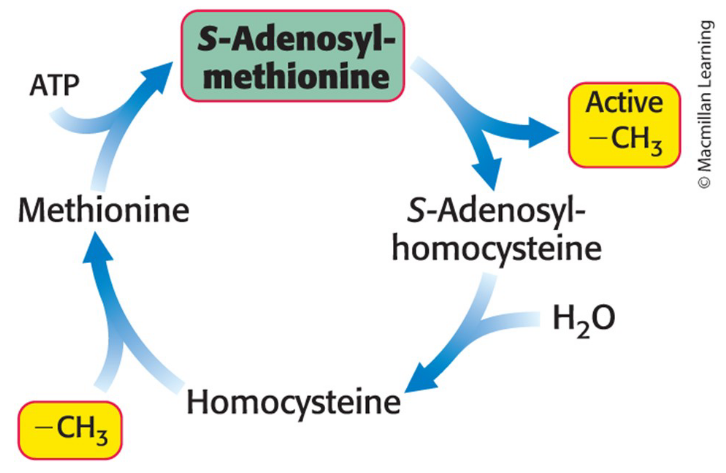
How is methionine used in amino acid biosynthesis?
S-adenosylmethionine (SAM) is a major donor for methyl groups so we synthesize it from methionine & ATP
SAM gets converted to homocysteine after donating a methyl group by S-adenylmethionine yielding homocysteine and adenosine
Can be used to regenerate methionine by a transfer of a methyl group
Mediated by coenzyme methylcobalamin
In order to keep reusing it
Make up activated methyl cycle
How is the synthesis of amino acids regulated?
Feedback inhibition: final product in a pathway inhibits enzyme catalyzing the committed step
Conserves building block and metabolic energy
Committed step in Ser synthesis - catalyzed by 3-phosphoglycerate dehydrogenase (which is inhibited by Ser)
Branched pathways are regulated by several feedback mechanisms:
feedback inhibition and activation: 2 pathways w/ a common initial step may each be inhibited by its own product and activated by the product of the other pathway
The mechanism functions to balance the amounts of different amino acids that are synthesized.
Ex: threonine deaminase = enzyme that catalyzes the formation of alpha-ketobutyrate
Allosterically inhibited by Ile (end product of its pathway) & allosterically activated by Val (end product of competitive pathway)
enzyme multiplicity: process by which the committed step can be catalyzed by two or more isozymes
Isozymes: enzymes w/ essentially identical catalytic mechanisms but different regulatory properties
Ex: phosphorylation of Asp, the committed step in the biosynthesis of Thr, Met, and Lys - catalyzed by 3 distinct aspartokinases
One is not subject to feedback inhibition.
One is inhibited by Thr.
One is inhibited by Lys.
cumulative feedback inhibition: process by which a common step is partly inhibited by multiple final products, each acting independently
Ex: glutamine synthetase
Glutamine is synthesized from glutamate, NH4+, and ATP.
The amide group of glutamine is a source of nitrogen in the biosyntheses of Trp, His, carbamoyl phosphate, glucosamine 6-phosphate, cytidine triphosphate, and AMP.
Glutamine synthetase is cumulatively inhibited by each of these final products of glutamine metabolism, as well as by Ala and Gly.