1) Cycloalkanes and their stereochemistry
1/10
Earn XP
Description and Tags
pages 1-2 in packet
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
What bond angles are typical for normal alkanes? Why?
109.5/ sp3/ this arrangement minimizes electron pair repulsions
Cyclopropane bond angle
60 degrees
Cyclobutane bond angle
90 degrees
Cyclopentane bond angle
108 degrees
Cyclohexane bond angle
120 degrees
The more a cycloalkane is farther from its ideal angle (109.5), what happens?
The energy will be higher and the molecule will be less stable
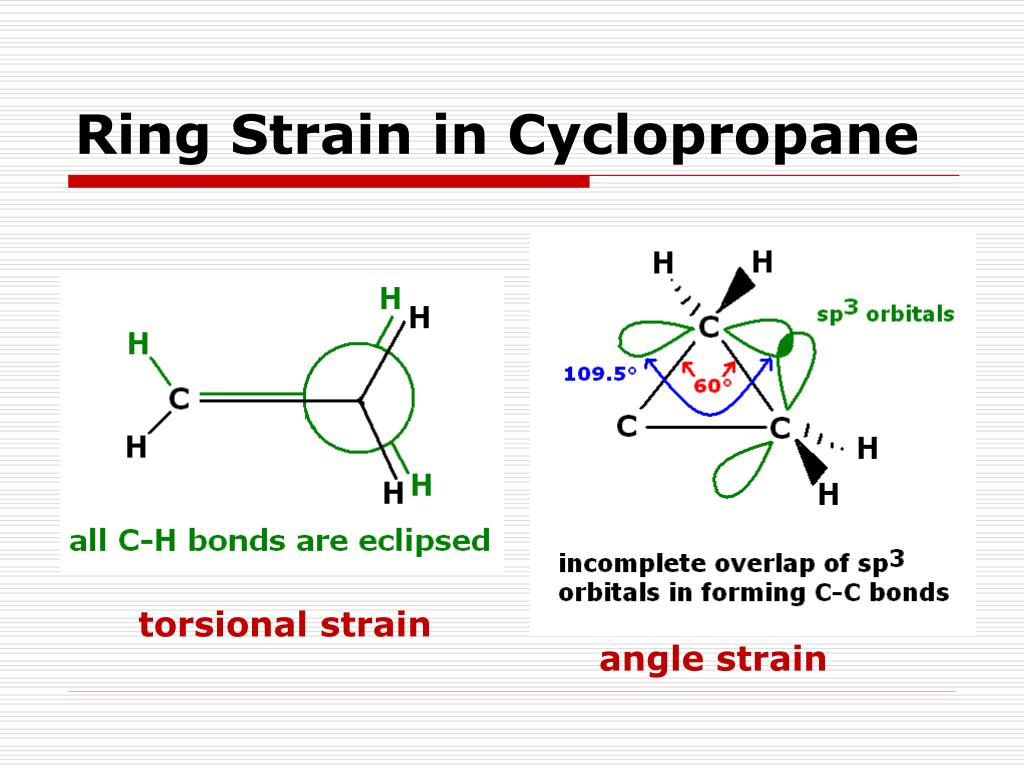
What is ring strain?
Combo of angle and torsional strain
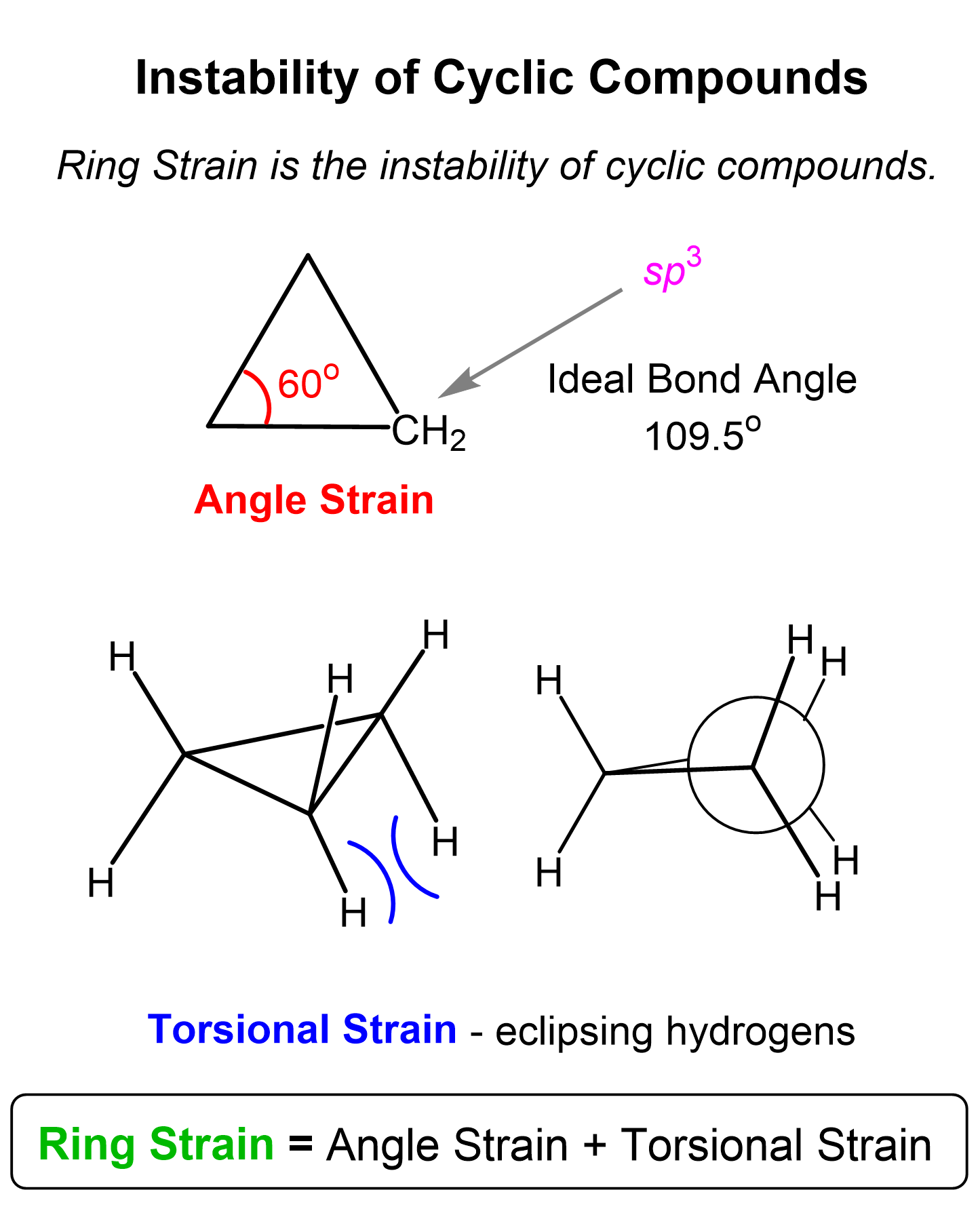
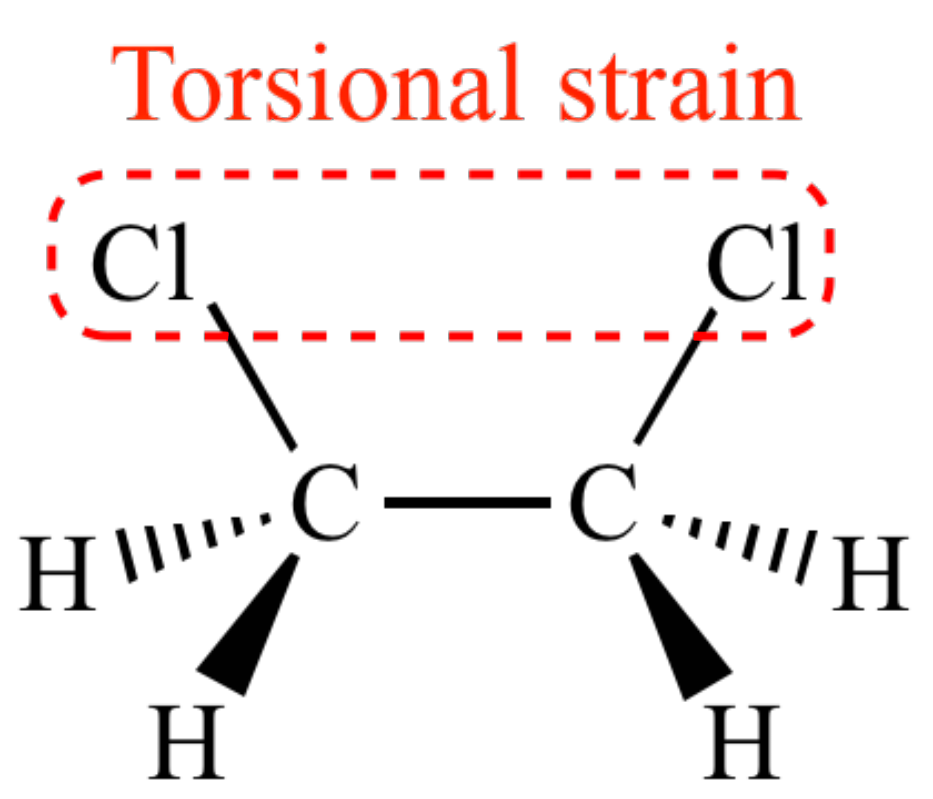
What is torsional strain?
when bonded atoms or groups are forced into eclipsed or gauche conformations, leading to electron repulsion between overlapping bonds/neighboring substituents. It arises due to restricted rotation in cyclic molecules.

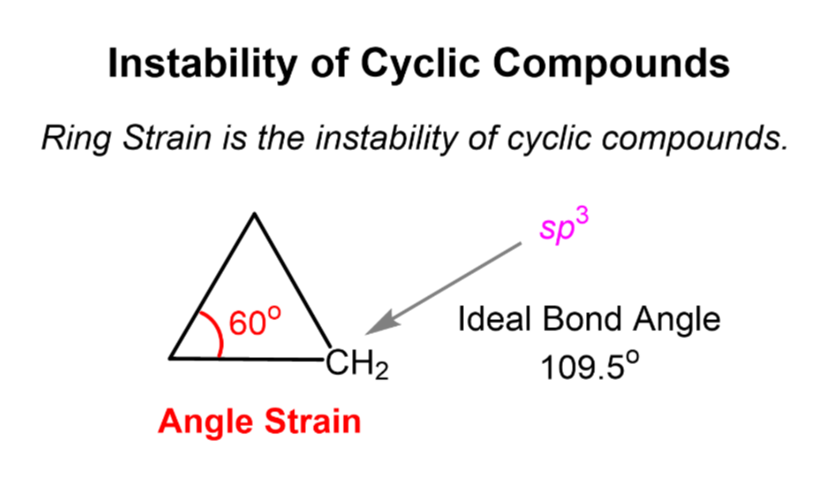
What is angle strain?
Occurs when bond angles deviate from the ideal 109.5° (for sp³ hybridized carbon).
Steric strain
What is torsional strain from chapter 3?
Resistance to bond rotation cause by repulsion between electron clouds