CHEM 4680 / Topic 4a: Toolbox - Substrate Activation
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
What happens in ligand substitution? What are the two kinds of ligand substitution?
Ligand replaces another ligand through two kinds: dissociative and associative.
Compare the associative and dissociative ligand substitution mechanisms in coordination complexes. Which electron counts favour each pathway and why?
• Ligand dissociates, leaving a vacant site at which the incoming ligand subsequently binds. Favoured by 18e complexes because if you add a ligand first, you reach a 20e int.
• Ligand binds to complex, then departing ligand leaves. Favoured by 16e complexes because you want to add a ligand first to get to an 18e int.
+ In dissociative, 16e int is reactive enough to add an extra ligand to get to 18e, but not too reactive to not form.
In a dissociative ligand substitution mechanism, what typically determines the rate of reaction, and how is the rate law expressed?
Ligand loss is usually slow followed by fast trapping of the vacant site by incoming ligand, so the RDS is usually independent of the concentration of the incoming ligand and dependent on the complex involved in dissociation.
rate= k1[LnM].
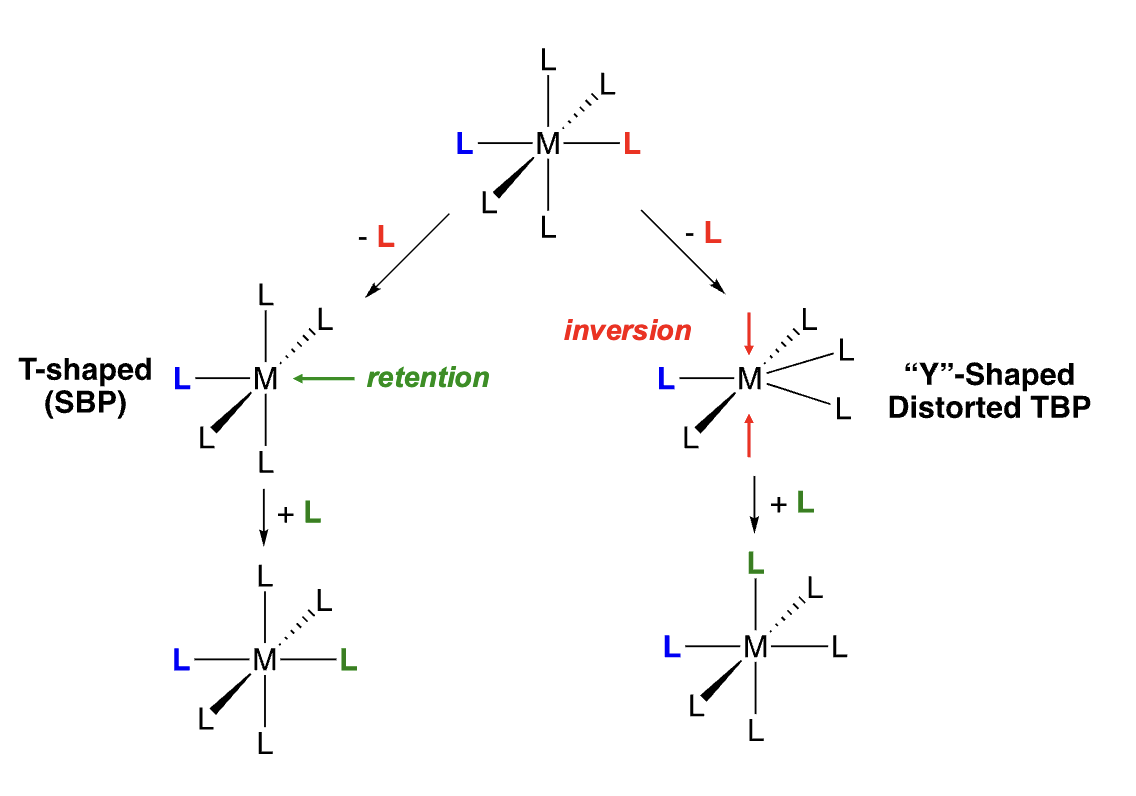
In dissociative ligand substitution of Oh complexes, what are the possible geometries of the intermediate, and how do they affect the stereochemical outcome?
There are two intermediates with two different geometries that can occur when dissociative substitution occurs.
> T-shaped or SBP for incoming ligand to land in between the two ligands out-of-axis. This leads to retention of configuration.
> Y-shaped distorted TBP for incoming ligand to land in between the equatorial ligand and one of one out-of-axis ligand. This leads to inversion of configuration.
What’s the order of relative energies of the d-orbitals in SBP, TBP, and Y-shaped distorted TBP?
SBP: z2 > x2-y2 > xy > xz,yz
TBP: z2 > x2-y2 and a close but lower-in-energy xy > xz,yz
Y-distorted TBP: z2 > xy > x2-y2 > xz,yz
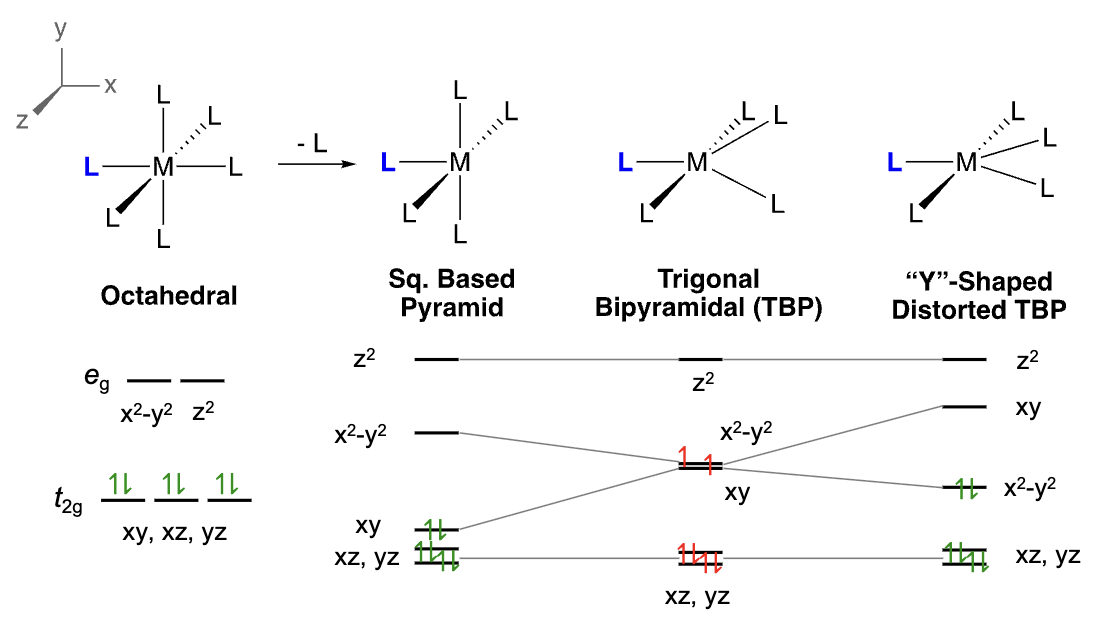
For paramagnetic d6 TBP complexes, why do they often distort to Y-shaped TBP?
(Jahn-Teller) Any non-linear molecule with will undergo a geometrical distortion that removes the degeneracy of unequally filled orbitals to lower the total energy of that molecule.
How do dissociative substitution rates vary across the 1st, 2nd, and 3rd rows of transition metals, and what is the underlying reason for these trends?
> 3rd row metals = Bigger and more diffuse orbitals = Stronger bonding with ligand = Slowest.
> 2nd row metals = Smaller orbitals = Weaker bonding with ligand = Slower.
> 3rd row metals = Smallest orbitals = Weakest bonding with ligand = Fastest.
How does steric bulk influence the rate of dissociative substitution?
> More bulky centre.
> Release of bulky ligand = Steric congestion relieved = Lower in energy = faster rate.
In an associative ligand substitution mechanism, what typically determines the rate of reaction, and how is the rate law expressed?
Ligand attack is usually slow, so the RDS is usually dependent of the concentration of the incoming ligand and the metal complex involved and independent on the dissociation of the ligand following association.
rate = k1[Lincoming][LnM]
How does the entropy of activation (∆S‡) differ between associative and dissociative ligand substitution mechanisms, and why?
• For associative, ∆S‡ is higher because, in the TS, everything has to be exactly ordered to accommodate a complex with a lot of ligands = requires more energy.
• For dissociative, ∆S‡ is lower because, in the TS, everything is less ordered and less accommodation because you’re removing a ligand from a complex = requires less energy.
What does the principle of microscopic reversibility state?
In a reversible reaction, the mechanism in one direction is exactly the reverse of the mechanism in the other direction, excluding reactions beginning with photochemical excitation.
What are the two requirements of a really good trans effect ligand?
• Strong σ donor:
• Strong π acceptor.
Why is a strong σ donor a good trans effect ligand in regards to M-Lsub strength?
> More electron-releasing / Less electronegative / More electropositive
> Energy of LTrans is higher
> More LTrans-like σ*
> Less metal-like σ*
> Less metal “attention” of σ* towards Lsub
> M-Lsub weaker
Why is a strong σ donor a good trans effect ligand in regards to repulsion?
> More electron-releasing / Less electronegative / More electropositive
> e density is pushed onto metal
> More repulsion with e density from Lsub.
What is the rs between electronegativity and trans influence?
EN ≈ 1/TI
What’s observable about the bond through X-ray crystallography between M-Lsub when a strong trans influence ligand is present?
Longer M-Lsub.
Why is a π acceptor a good trans effect ligand?
> Stronger M-Ltrans interaction.
> Less attention of M to Lsub.
> Weaker M-Lsub.
In a TBP complex with three π-acceptors in the equatorial position, which dπ will be the most π-basic, and why?
xy will be most basic, as its HOMO is higher in energy than the other π-basic sites xz and yz.
> Higher HOMO.
> Better bonding.
> Better backbonding.
Differentiate trans effect and trans influence.
Trans effect: Kinetics-related, rate affected by the trans influence.
Trans influence: Thermodynamics-related, ground state is affected by energetic properties.
How can an 18e complex undergo associative substitution without forming an unfavourable 20e int?
18e complexes can undergo "associative" substitution without forming unfavourable 20e int if a ligand can rearrange to produce a 2e "vacancy" to enable binding of an incoming ligand, e.g. cyclopentadienyl complexes.
What occurs in transmetallation?
Swapping of a metal of an alkyl and a metal of a halide.
What are the requirement(s) in transmetallation?
> Ms-R, where R is a carbon-containing group and Ms is a s-block metal.
> Md-X, where Md is a d-block metal.
Why is the identity of the main-group reagent important in transmetallation? On this note, why are Suzuki couplings more functional group tolerant?
> The more electropositive Ms is, the more reactive the reagent due to ∆EN, meaning it will be more reactive in transmetallation and other side rxns.
> The more oxidized Md is, the more reactive it then is for similar reasons.
> Suzuki couplings incl. organoboron reagents, and with a less δ- carbon or less nucleophilic carbon, you can have some fxnal group tolerance.
Why is the identity of the R group important in transmetallation?
> The more basic the R group, the more reactive it is, e.g. alkyl > aryl/benzyl > alkynyl.
> Bulky R = Sterically hindered metal = Not going to react efficiently.