Organic chemistry-carbonyls
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
19 Terms
what is sterioisomerism?
Molecules with the same structural formula, but a different 3-D arrangement/arrangement in space
what are the two types of sterioisomers?
E/Z or cis/trans
Optical isomerism: occurs due to the presence of a chiral carbon. Object and its mirror image.
what are optical isomers called?
Enontiomers - are is non-superimposable
A mixture containing an equimolar amount of the two isomers is called a racemix mixture
what are optical isomers/enontiomers
these are non-superimposable mirror image of each other. The central atom is a chiral centre/carbon
what is a chiral
A chiral carbon has four different atoms, or groups of atoms bonded to it
Often referred to as asymmetric carbons
Both the bonds and the atoms are mirro images
Properties of optical isomers
they have identical physical and chemical properties but with 2 exceptions:
They rotate the plane of polarised light in opposite directions ie they are optically active
they interact differently with other optically active molecules. They are sterioselective.
Optical isomers interact with biological sensors in different ways
For example, one enantiomer of carvone smells of spearmint, while the other smells of caraway
how to determine the identity of an optical isomer of a single substance
The rotation of plane polarised light can be used to determine the identity of an optical isomer of a single substance
For example, pass plane polarised light through a sample containing one of the two optical isomers of a single substance
Depending on which isomer the sample contains, the plane of polarised light will be rotated either clockwise or anti-clockwise by a fixed number of degrees
what mechanisms do primary halogenoalkanes and tertiary halogenoalkanes use to react?
Primary halogenoalkanes react via Sn2 mechanisms
Tertiary halogenoalkanes react via Sn1 mechanisms
what are SN1 and SN2 mechanisms?
SN1: Substitution, nucleophile, 1=unimolecular so 1 molecules in RDS
SN2: substitution, nucleophilic, 2=biomolecular so 2 molecules in rds
what is a racemix micture
A racemic mixture (or racemate) is a mixture containing equal amounts of each enantiomer
One enantiomer rotates light clockwise, the other rotates light anticlockwise
A racemic mixture is optically inactive as the enantiomers will cancel out each others effect
This means that the plane of polarised light will not change
how racemix mixtures used in drugs
In the pharmaceutical industry, it is much easier to produce synthetic drugs that are racemic mixtures than producing one enantiomer of the drug
Around 56% of all drugs in use are chiral and of those 88% are sold as racemic mixtures
Separating the enantiomers gives a compound that is described as enantiopure, it contains only one enantiomer
This separation process is very expensive and time consuming, so for many drugs it is not worthwhile, even though only half the of the drug is pharmacologically active
For example, the pain reliever ibuprofen is sold as a racemic mixture
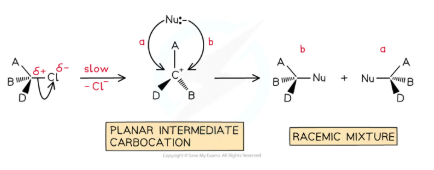
are SN1 mechanisms optically active?
the reactant is optically active but product is not
In the first step, the C-X bond breaks heterolytically and the halogen leaves the halogenoalkane as an X- ion
This leaves a trigonal planar, tertiary carbocation
In the second step, the planar, tertiary carbocation is attacked by the nucleophile
The OH- attacks the intermediate randomly from the right or the left. An equal number of each optical isomer is formed which results in racemix mixture
is SN2 mechanism optically active?
The SN2 mechanism is a one-step reaction
The nucleophile donates a pair of electrons to the δ+ carbon atom of the halogenoalkane to form a new bond
At the same time, the C-X bond is breaking and the halogen (X) takes both electrons in the bond
The halogen leaves the halogenoalkane as an X- ion
Optically active at start to optically, but in the oposite direction
The bromine atom of the bromoethane molecule causes steric hindrance
This means that the hydroxide ion nucleophile can only attack from the opposite side of the C-Br bond
Attack from the same side as the bromine atom is sometimes called frontal attack
While attack from the opposite side is sometimes called backside or rear-side attack
As the C-OH bond forms, the C-Br bond breaks causing the bromine atom to leave as a bromide ion
As a result of this, the molecule has undergone an inversion of configuration
The common comparison for this is an umbrella turning inside out in the wind
products optically active but in the opposite directions
what are carbonyls?
Functional group C=O
Aldehydes and ketones and functional groups
Aldehydes have C=O at end of a chain
Ketones have C=O not on first/last, in middle
intermolecular forces of carbonyls
Aldehydes and ketones have a dipole within their structure due to the electronegative oxygen of the carbonyl group. C=O bond is polar.
This means that aldehydes and ketones have permanent dipole-dipole interactions and London forces between molecules
distinctive proprties of aldehydes and ketones
they have distinctive smells
boiling points of aldehydes and ketones?
Aldehydes and ketones have lower melting and boiling points than alcohols with a similar mass
they have permanent dipole-permanent dipole interactions, rather than hydrogen bonds
However compared to alkanes with a similar mass, their boiling points will be higher, as pd-pd forces are stronger than London forces
Solubility of carbonyls?
Although carbonyls cannot hydrogen bond with themselves, they do have the lone pairs on their oxygen
This means that smaller aldehydes and ketones are able to hydrogen bond with water
The δ– oxygen atom from the carbonyl uses its lone pairs to form hydrogen bonds with the δ+ hydrogen from water
As a result short chain carbonyls are soluble in water
but longer the chain length, the less soluble it becomes
the hydrocarbon chains form London forces that require more energy to be overcome.
Larger aldehydes and ketones have longer hydrocarbon chains which also cannot hydrogen bond with water
These hydrocarbon chains can disrupt the hydrogen bonding within water but cannot form hydrogen bonds themselves
what is needed for substance to dissolve
In order to dissolve, the strength of the potential hydrogen bonding of the carbonyl with water must be higher than the combined strength of the intermolecular forces of the carbonyl and the hydrogen bonding of water