Science Validation Reaction Rate
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
25 Terms
State the collision theory
Collision theory states that for a chemical reaction to occur, particles must:
Collide with each other
Have sufficient kinetic energy to overcome the activation energy barrier
Collide with the correct orientation
Only collisions that meet all three conditions result in a successful reaction. This explains why increasing temperature, concentration, or surface area can speed up a reaction — they increase the number of effective collisions.
What is the rate of reaction?
A measure of how quickly a chemical reaction occurs. It tells you how fast reactants are used up or how fast products are formed over time.
Describe how rate of reaction can be determined.
Measuring the rate of a reaction means measuring the change in the amount of a reactant or the amount of a product.
Rate of reaction = change in concentration / change in time
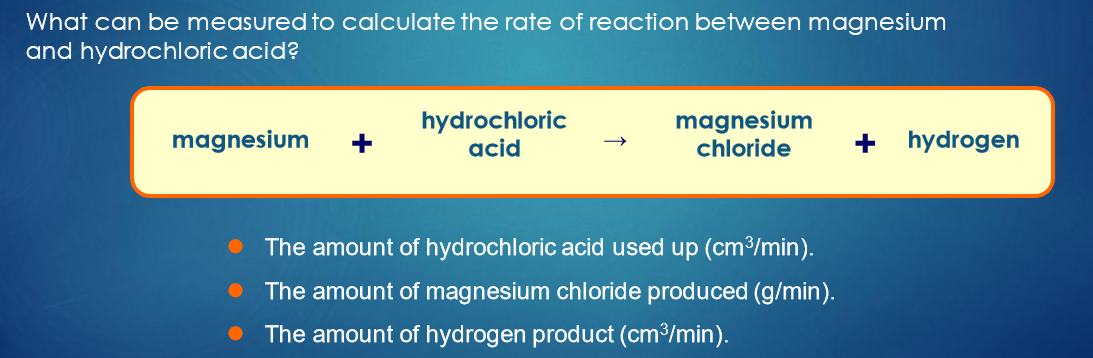
Describe the graph that shows how reaction changes over time
Reactant line (blue):
Starts high and curves down → reactants are used up over time.Product line (brown):
Starts at 0 and curves up → products are formed as reaction progresses.Both lines flatten out when the reaction is complete (no more changes in concentration).
What is activation energy?
The minimum amount of energy needed for the particles to react.
What 2 things do the rate of a reaction depend on?
The frequency of collisions between particles
The energy with which the particles collide
If the particles collide with less energy than the activation energy, they will not react. Instead, they will bounce off of each other.
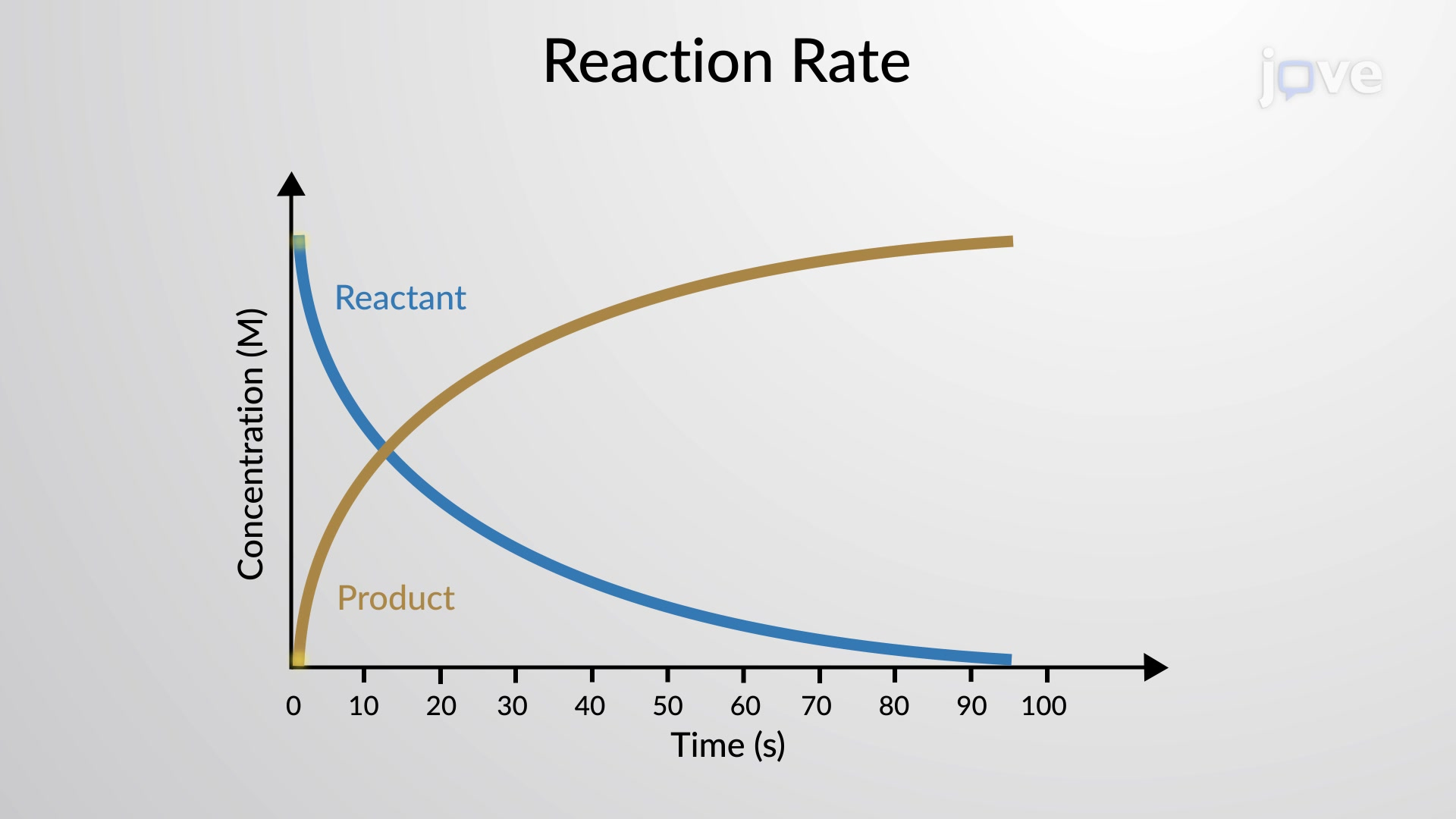
How does temperature impact the rate of a reaction?
Higher temperature = faster the rate of a reaction (as the particles move at a faster speed)
A rise in 10 deg usually causes the rate of a reaction to approximately double
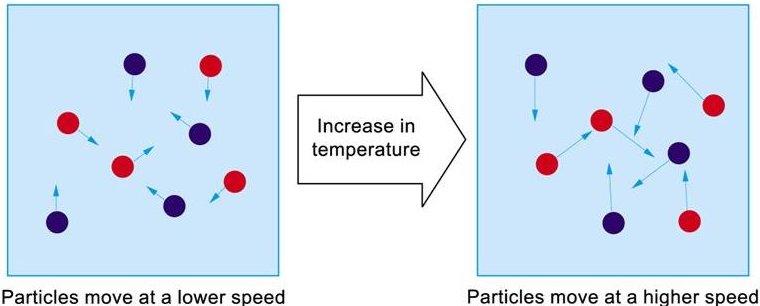
How does concentration impact the rate of a reaction?
Higher the concentration of a dissolved reactant = the faster the rate of a reaction
At a higher concentration, there are more particles in the same amount of space.
This means that the particles are more likely to collide and therefore more likely to react.
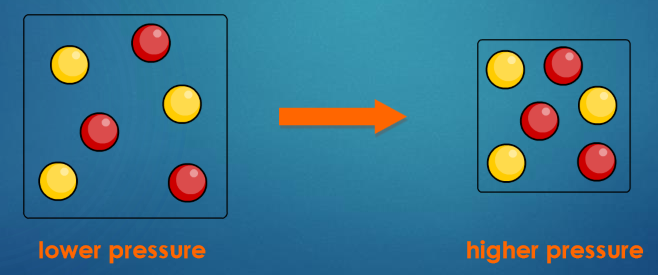
How does pressure impact the rate of a reaction?
The pressure of gaseous reactants increase the rate of a reaction
As the pressure increases, the space in which the gas particles are moving becomes smaller.
The gas particles become closer together, increasing the frequency of collisions,. This means that the particles are more likely to react.
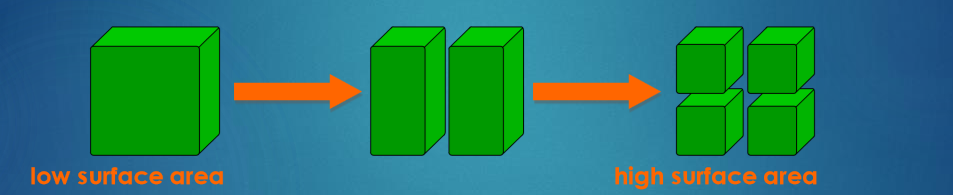
How does surface area influence the rate of a reaction?
Any reaction involving a solid can only take place at the surface of a solid
If the solid is split into several pieces, the surface area increases.
This means that there is an increased area for the reactant particles to collide with.
The smaller the pieces, the larger the surface area. This means more collisions and a greater chance of reaction.
What is a catalyst?
Substances that change the rate of a reaction without being used in the process.
Catalysts never produce more product - they just the produce the same amount more qucikly
Different catalysts work in different ways, but most lower the reaction’s activation energy.
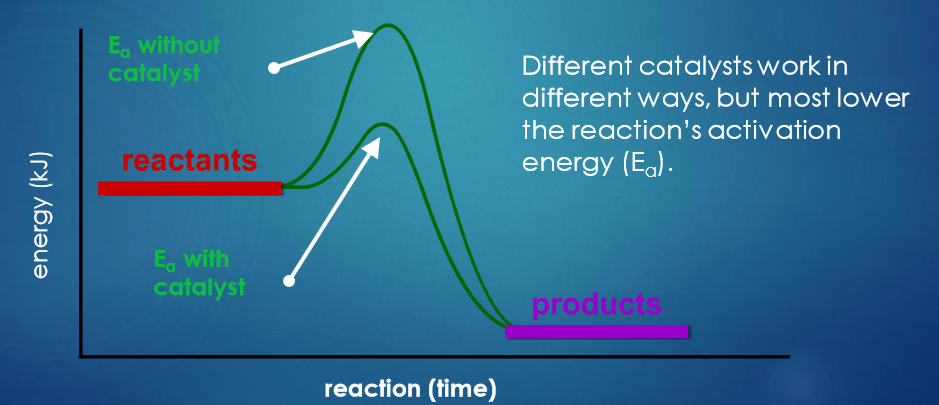
How does an enzyme lower the activation energy of a reaction?
Catalyst lowers activation energy by providing an easier route for the reaction.
This makes it quicker for reactants to turn into products.
Explain the affect on reaction rate using collision theory.
More frequent collisions → faster reaction.
Higher energy (e.g., higher temp) → more successful collisions → faster reaction.
Correct orientation needed for reaction.
Higher concentration or pressure → more collisions → faster reaction.
Catalyst lowers activation energy → more successful collisions → faster reaction.
How does increasing temperature, concentration, surface area, or adding a catalyst affect reaction rate?
Increasing temperature: Reaction rate increases.
Increasing concentration of reactants: Reaction rate increases.
Increasing surface area of reactants: Reaction rate increases.
Adding a catalyst (or enzyme): Reaction rate increases.
Compare the two types of data
Quantitative Data – measurements written as numbers with units attached to them.
Qualitative – Data describe in words.
What are mistakes?
Can be avoided
E.g.
Spilling chemicals
Using the wrong equipment
Using the right equipment incorrectly
Reading instruments incorrectly
Copying measurements down incorrectly
What are errors?
Not mistakes
Small and unavoidable variations that occur naturally in
measurements.
E.g.
Parallax error
Instrument error
Human reflex
Zero error
How to improve primary data?
Repeat your measurements.
Ignoring outliers and calculating the average (mean) of the remaining data.
What is reliability?
An experiment is considered reliable if it gives the same results every time it is performed.
Reliability of results can be increased through:
Repeating trials – doing the same experiment many times to ensure that the results can be reproduced.
Replication – duplicating experimental set-ups, so that the experiment can be run more than once at the same time. (Duplicate experiments are called replicates)
Large sample size – having a large number of subjects/participants
What is validity?
An experiment is considered valid if it accurately tests the hypothesis and only the independent variable is changed.
Validity of results can be increased through:
Tightening the control variables
Improve experimental design
Validity vs Reliability
Reliable = consistent results
Valid = correct test of the aim
What are ethics?
Set of moral principles or values.
Ethical behaviour – behaviour that follows those principles or values.
Investigations involving humans must satisfy the following ethical principles:
Voluntary participation – don’t pressure participants to become involved.
Informed consent – fully inform participants about the objectives of the research/procedures to be followed/possible risks/potential benefits; consent should be sought after all information has been given.
Risk of harm – no risk or physical or psychological harm to participants
Confidentiality – identities will not be revealed except to people directly involved in the study.
Right to withdraw – participants can leave an experiment at any time without reason
What are the three types of variables?
Dependent - What is being measured
Independent - What is being changed
Control - What stays the same
What is molarity? (probs not needed)
It tells you how many moles of solute are dissolved in 1 litre of solution.
Molarity (M)=llitres of solution/moles of solute
Why does a higher concentration (e.g. 0.250 M) make a precipitation reaction faster than a lower concentration (e.g. 0.075 M)?
A higher concentration means more ions are present, causing more frequent collisions between reactant particles.
This leads to the precipitate forming faster.