AS Chemistry Formulae
0.0(0)
0.0(0)
Card Sorting
1/4
Earn XP
Description and Tags
AS OCR A
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
5 Terms
1
New cards
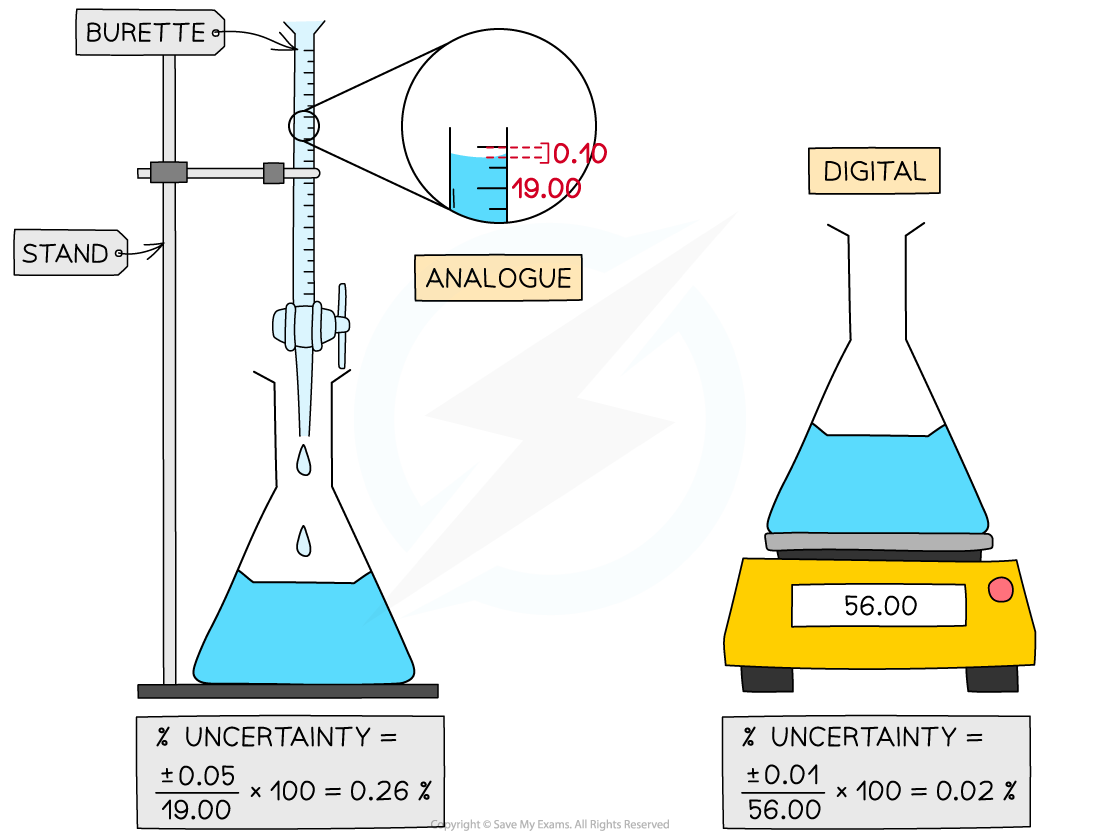
Percentage uncertainty formula
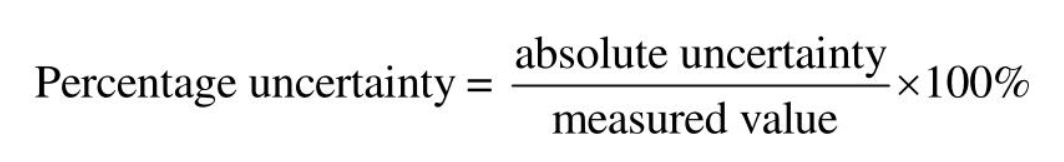
2
New cards
Percentage error formula
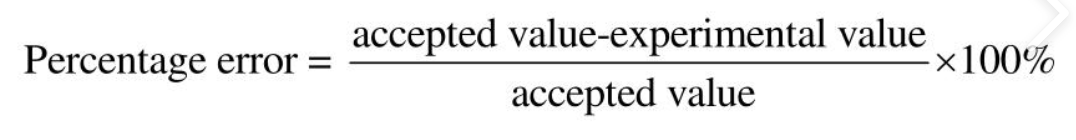
3
New cards
Total percentage uncertainty method
Add up all uncertainties of individual apparatus
4
New cards
Multiple readings uncertainty method
e.g. finding initial and final mass or temperature
DOUBLE the absolute uncertainty e.g. (2×±0.05)/25.00
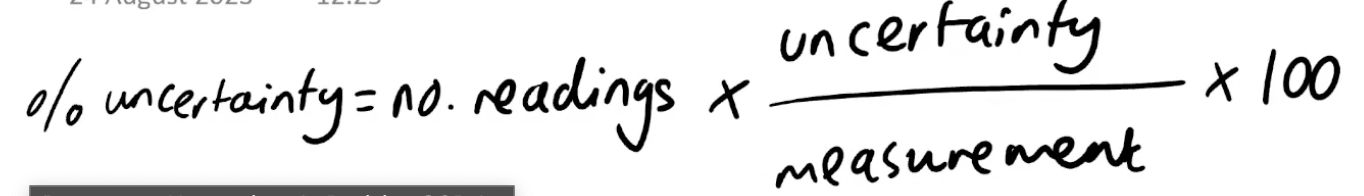
5
New cards
Dividing or multiplying measurements uncertainty method
e.g. finding rate of reaction (rate=vol/time)
add together percentage uncertainties
THEN calculate absolute uncertainty from sum of percentage uncertainties