L6: Nuclear transport, import and export
1/53
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
54 Terms
Why is DNA kept in the nucleus
Protect from harmful molsules→ e.g ROS from the mitochdonria
Protect from foreign DNA (virus etc)
if the cells knows that their own DNA is in the nucleus
when there is foreign DNA in the cytoplasm
can be sure and be harsh with the foreign DNA
to get rid of it
Extra regulation
gives a gate for transiption factors
TFs can be in nucleus or outside nucelus
do not need to be constantly brokendown/made again
if it is regulated by the entry into the nucleus
Nuclear disassembly and re-assembly during mitosis: in higher eukaryotic cells, when does nuclear disassembly take place
in prophase of mitosis
following complete chromosome replication in the previous S phase
Nuclear disassembly and re-assembly during mitosis: Process of nuclear disassembly
Increased protein phosphorylation → by cyclin B-CDK1
result in nuclear envelope breakdown
lamna repolymerises into
soluble lamin A/C
membrane assoaciate lamin B
Nuclear pore complexes (NPCs) disassemble into soluble nucleoporin subcomplexes
nuclear membranes fragment into vescicles or tubes
chromatin condenses into separated chromosomes until metaphase
associates with spindle apparatus
during this period: chromatin is directly accessible to the cytoplasm
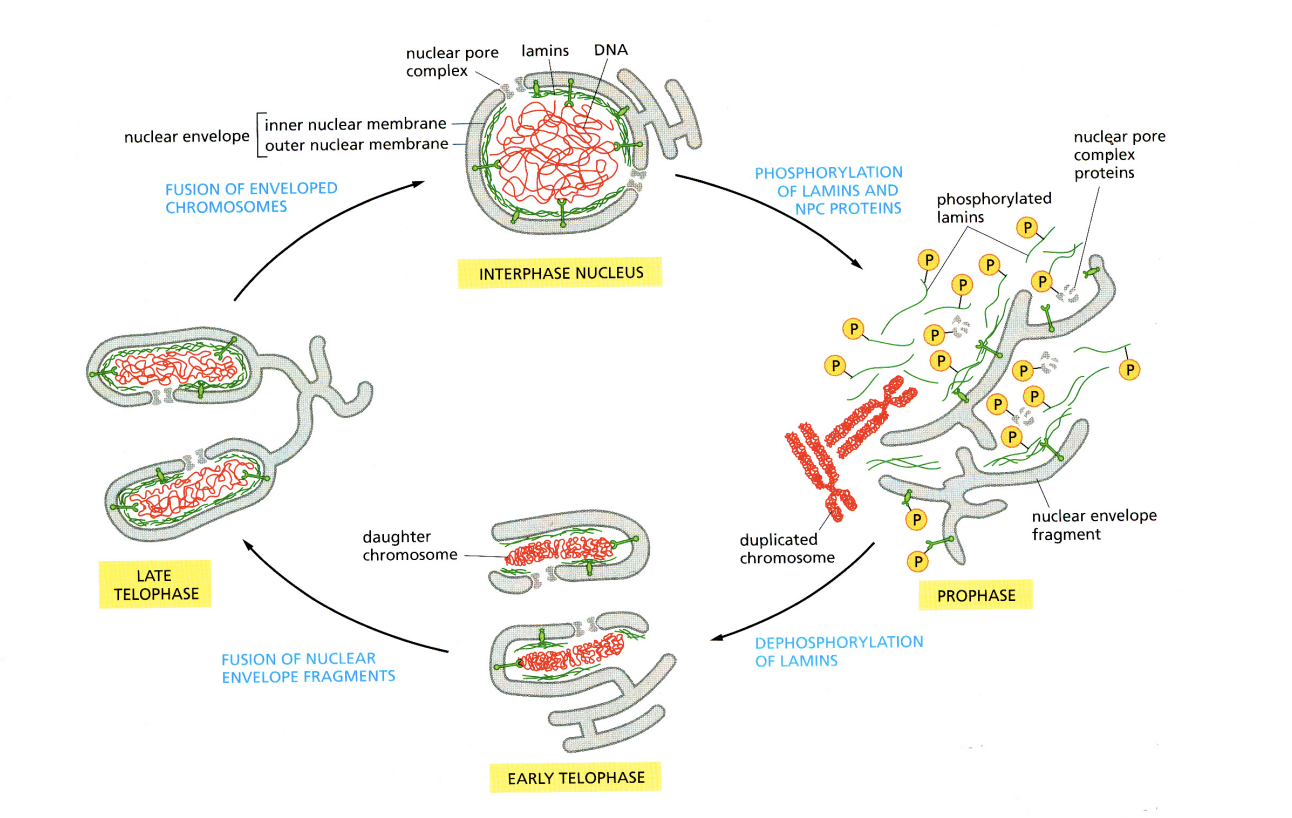
Nuclear disassembly and re-assembly during mitosis: following inactivation of CDK-complexes and a sharp fall in kinase activity in anaphase…
lamins and NPC proteins become dephosphorylated
→ causes nuclei re-assembly
What does this cause: Nuclei re-assembly
Reassemble in telophase after successful sister chromatid separation
→ inversion of the disassembly process involving:
chromosome decondensation
membrane assembly from vescicles
lamina polymerisation
NPC assembly from soluble nucleoporin subcomplexes
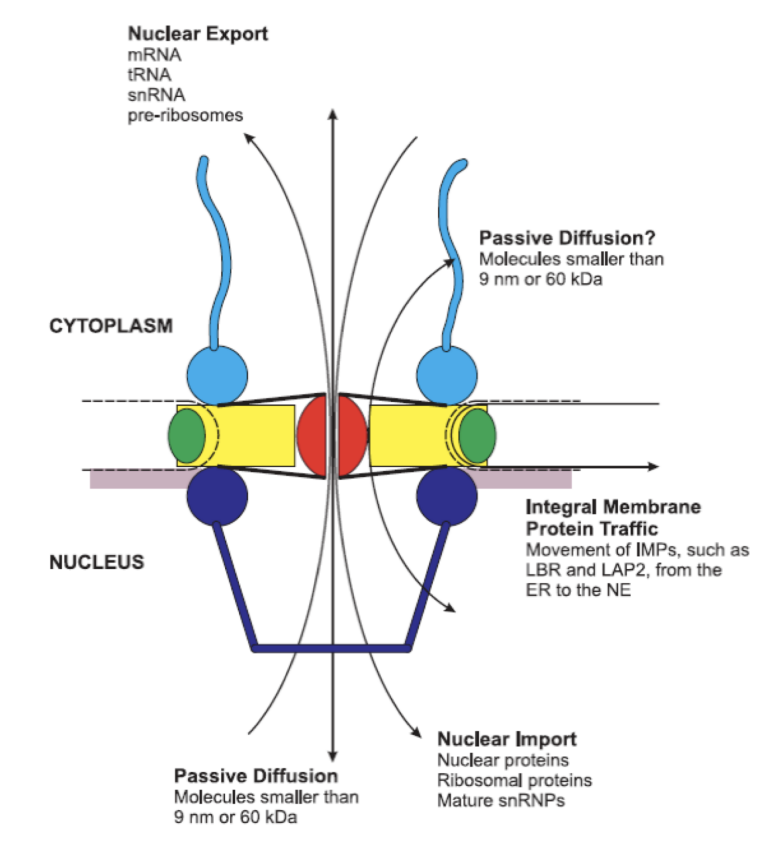
But now the nuclei have reassembled→ what must happen next?
several million proteins and RNA molecules must travel across the nuclear envelope per minute during interphase
All mediated by nuclear pore complexes…how?
Transport acrosss the nucler envelope
nuclear portins
highly conserves
rigid strucutre→ opens up gate
Passive diffusion→ only with smaller than 9nm or 60kDa
Everything bigger needs energy and help
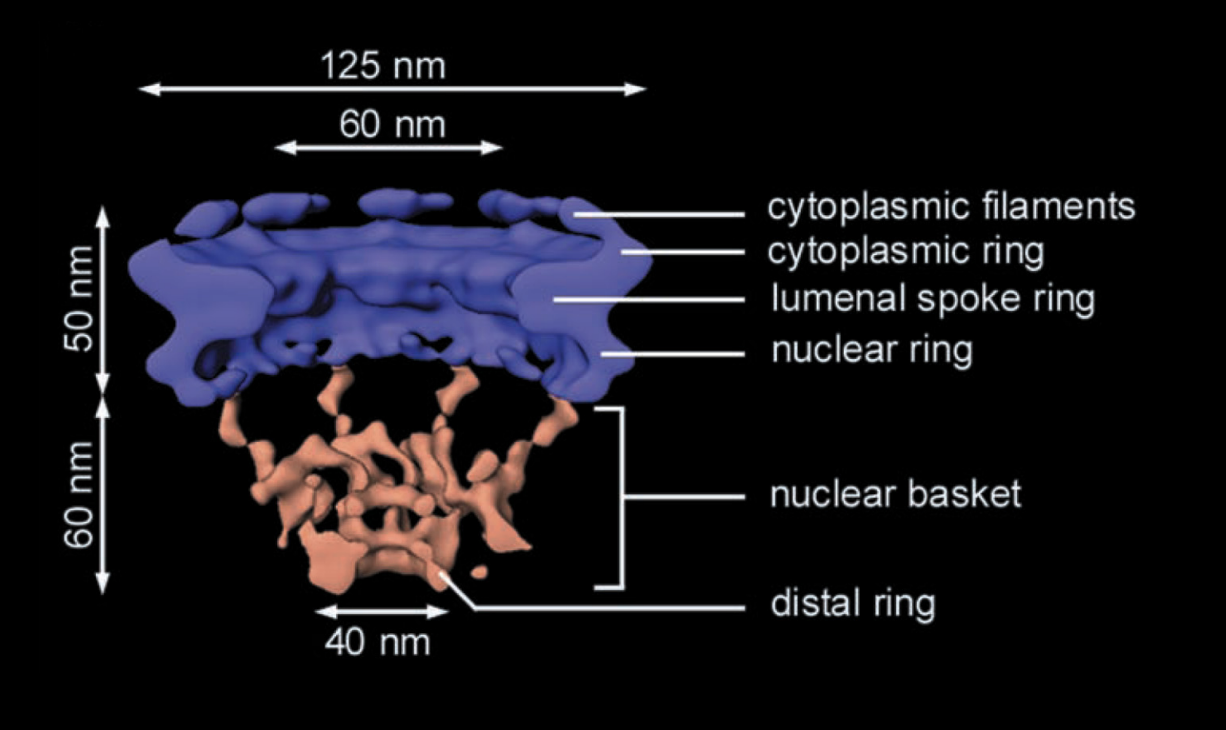
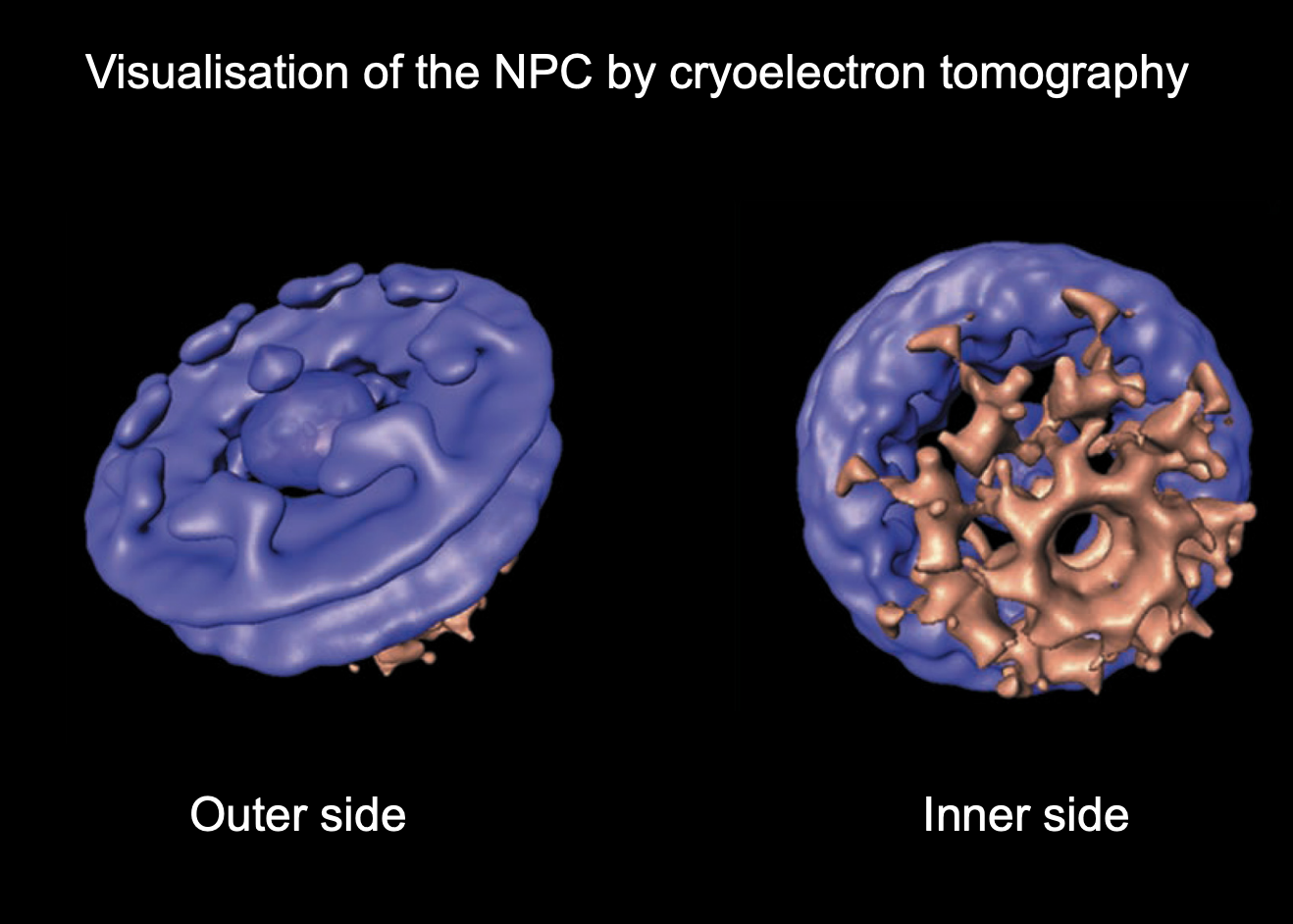
Nuclear pore complexes: strucutre (from EM)
ring of 8 subunits surrounding a central channel
through which proteins and RNA pass
with cytoplasmic filamers→ collapses from the cytosplasm>
8 subunits of NULCEOPORINS
subunits have different proteins themselves
fibrils project from both surfaces of the nuclear pore complex
those on the inside are organised as basket
Well maintained (also called a cage)
What occupies the central channel (gate) through the NPC
The nuclear pore is 40nm BUT only 9nm can diffuse through→ MUST be some kind of gate in the centre:
Protein structure lining and occupying the central gate of the NPC:
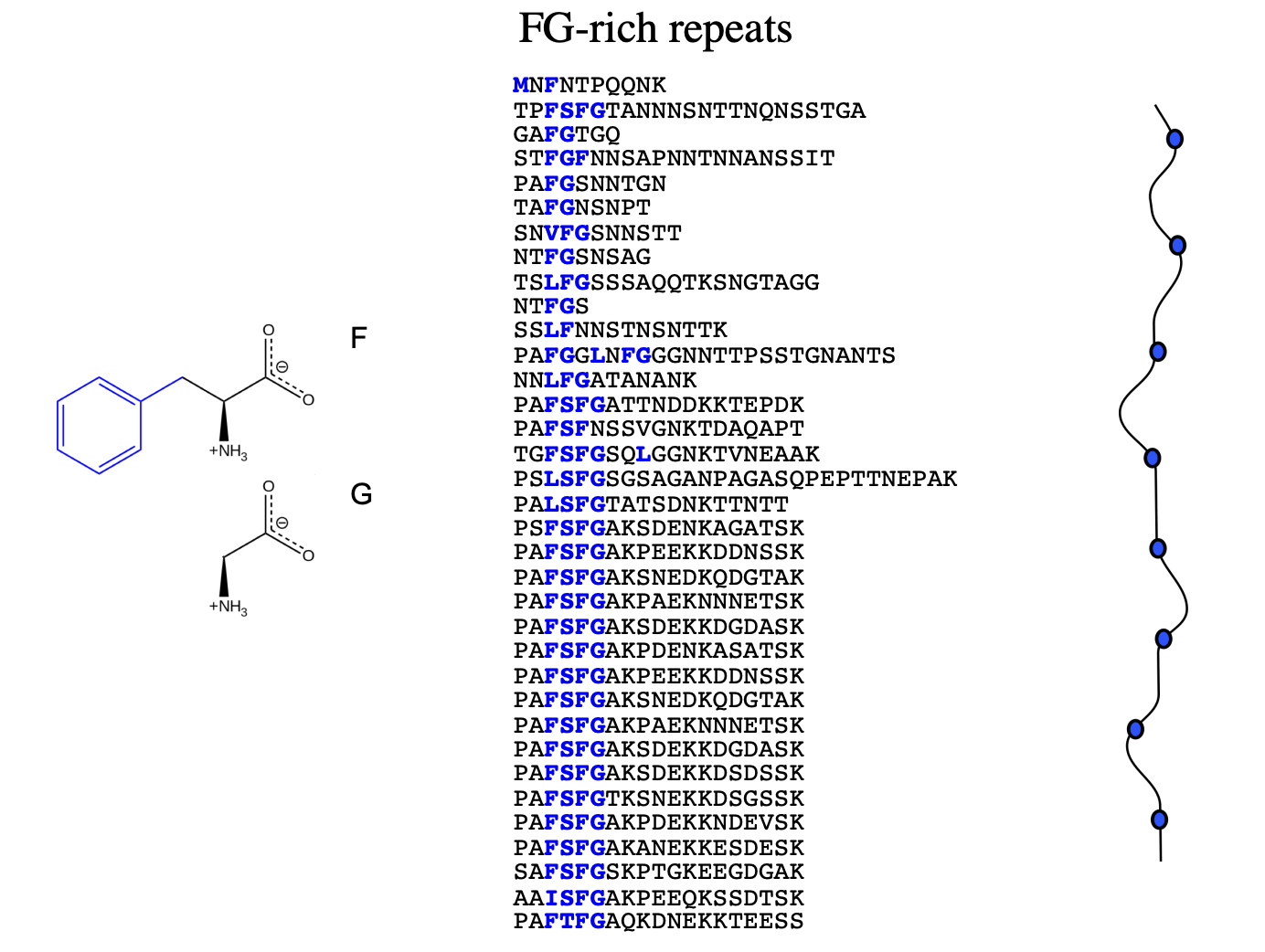
consists of nucleoporin proteins
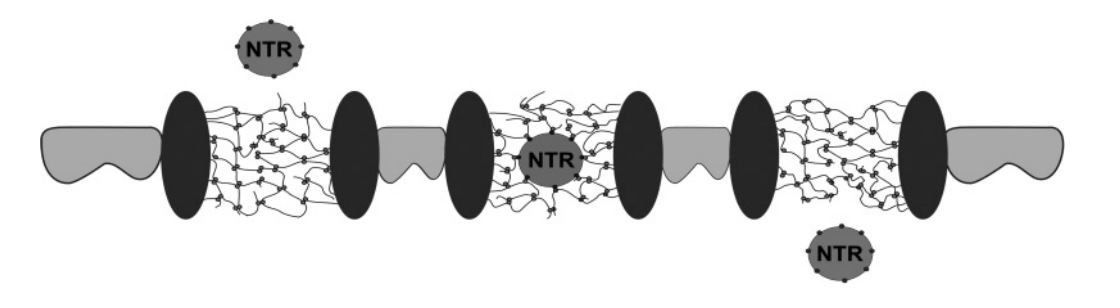
very rich in repeats f the 2 hydrophobic amino acids phenylalanine and glycine (FG)
FG repeats can interact with each other and form a dynamic hydrogel
there are several models postulated about this strucutre
OUTER→ has a central gate n the middle→ somehthing blocking entry/exit
INNER→ shows the rigid opens in the inner side of the basket
What is the gate?
selectivity barrier for macromolecules
→ assembled from nucleoporin proteins containing extended domains with FG repeats
→ have FG (phenylalinine and glycine) rich repeats
forms hydrophobic (blue blobs) along the protein
at irregular intervals
Next question to ask
how can interaction between the translocating proteins
and the F/G rich repeats favilitate NPC passage
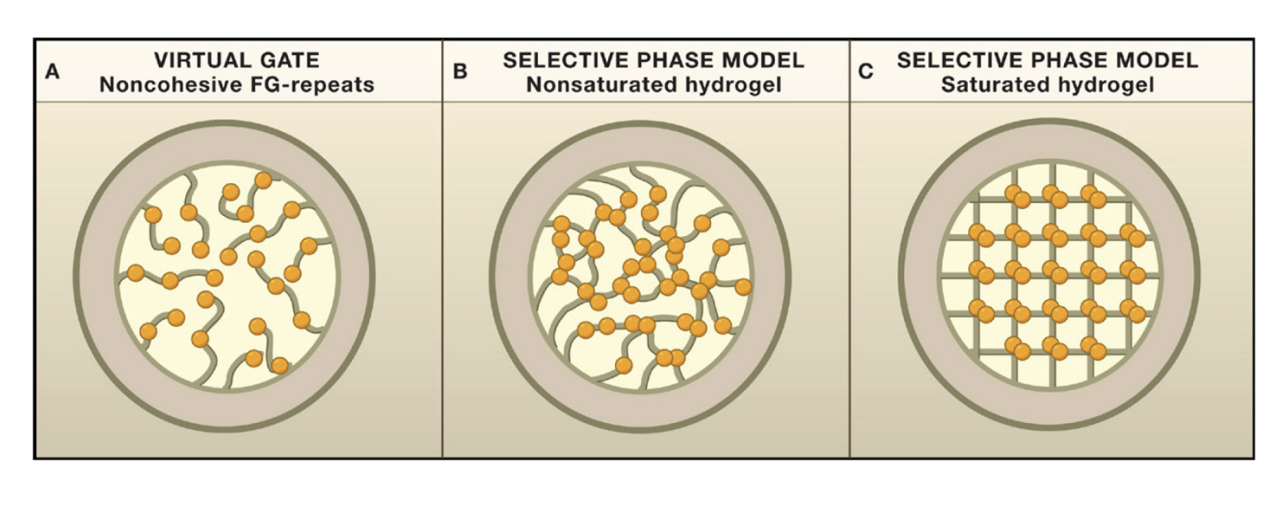
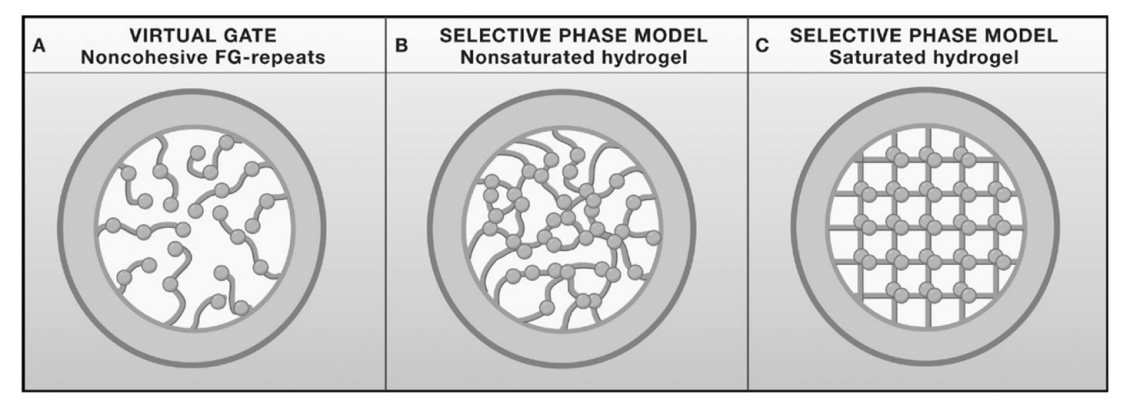
Several models of how synamic hydrogel forms: Virtual gate
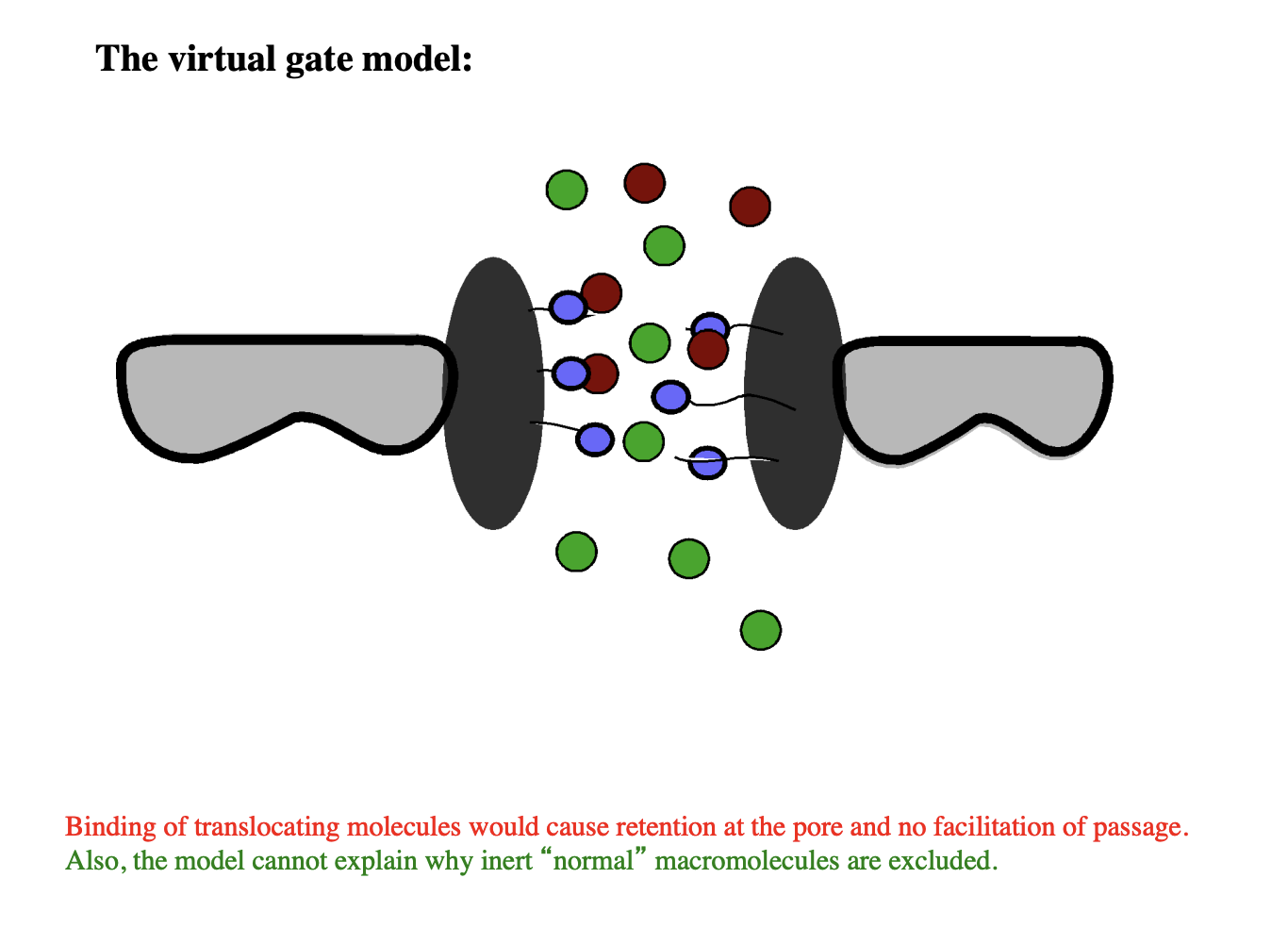
Several models of how synamic hydrogel forms: how does virtual gate model work?
green→ macromolecules
red→ translocating molecules, interacting with FG repeats
RESULT: does not show selective transport
CANNOT BE THE CORRECT MODEL
Several models of how synamic hydrogel forms: how might the hydrophobic blobs interact
formation of a hydrogel
→ leads more to the selective phase model
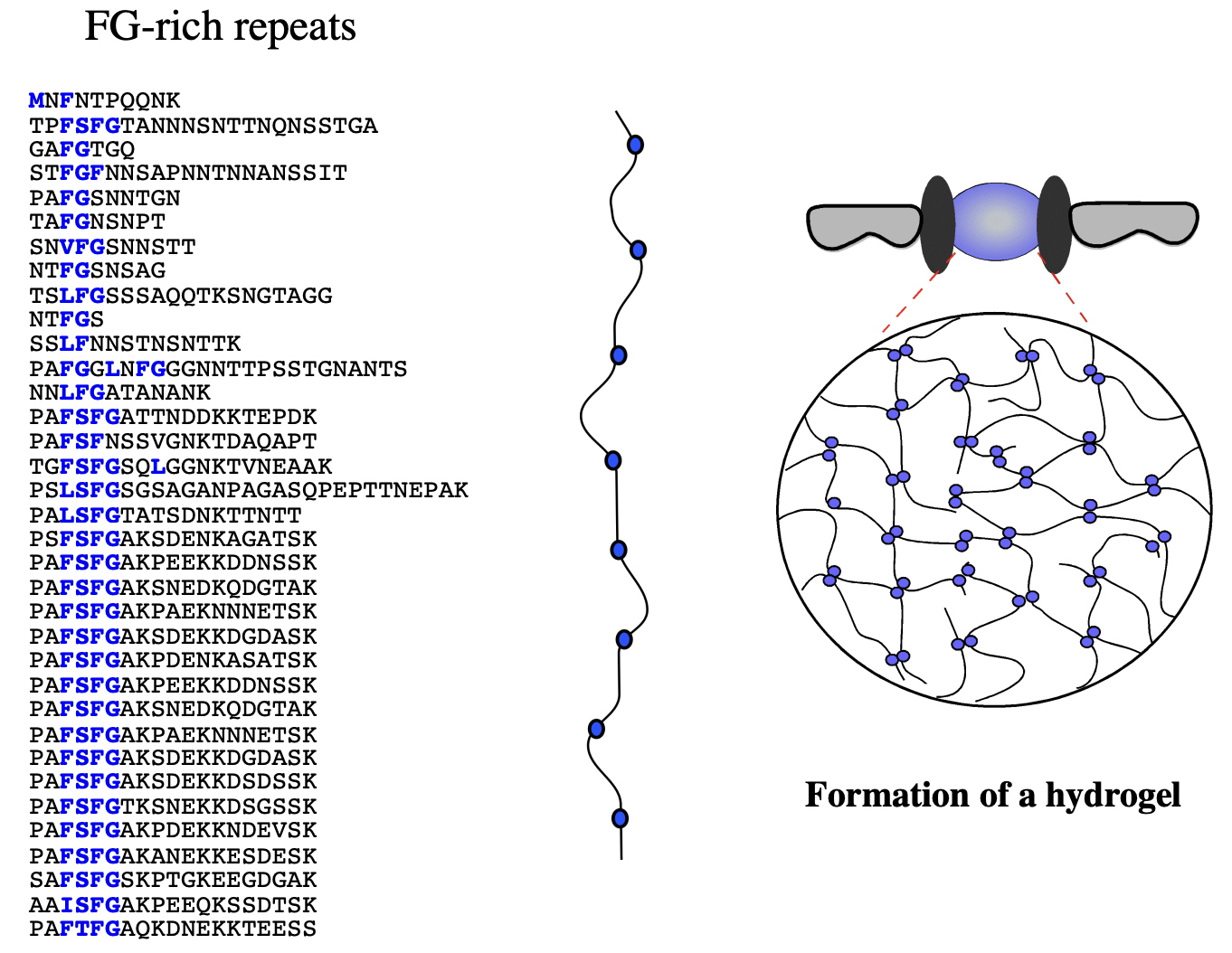
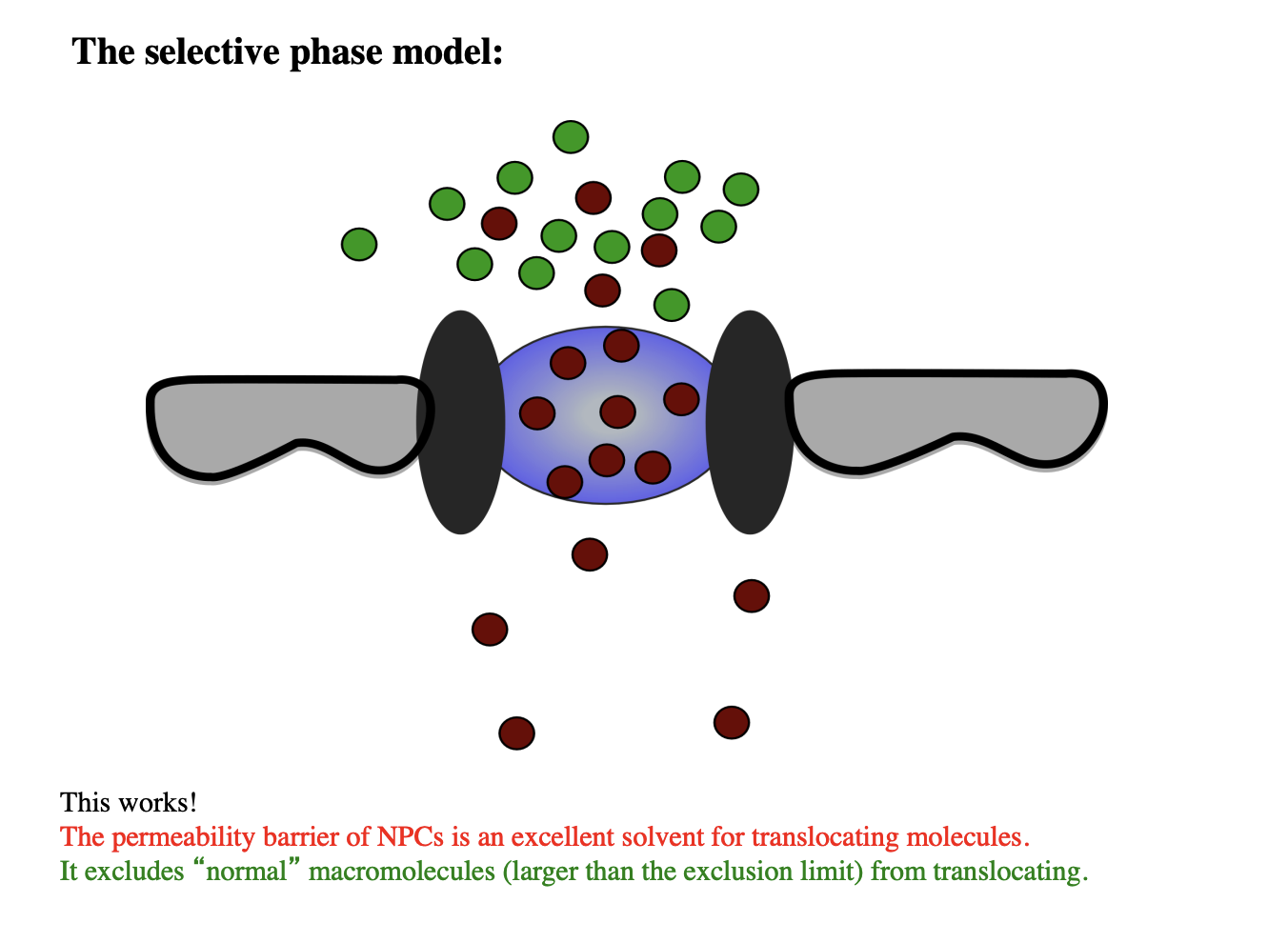
Several models of how synamic hydrogel forms: how the selective phase model
red→ blocks pore
green→ no selectivty
RESULT: selective importation
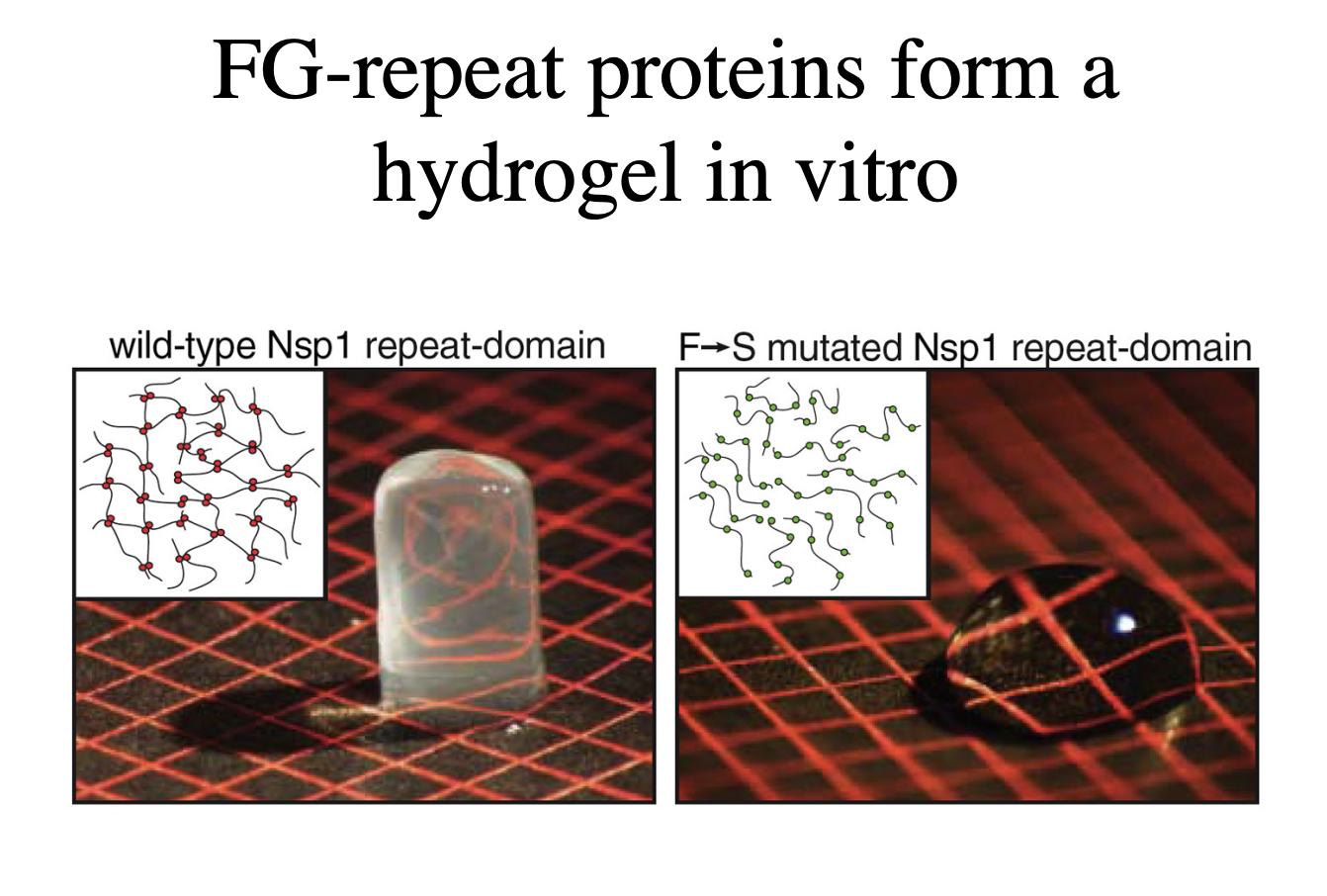
Several models of how synamic hydrogel forms: experimental evidence for this
forming the Nsp1 repeat domain
if mutated protein→ no longer a gel!
RESULT: must be true
Several models of how synamic hydrogel forms: what phase model is favoured
SELECTIVE PHASE MODEL:
hydrogel of cohesive FG-repeat proteins
form a barrier against diffusion of general marcomolecules
whilst providing a solvent for translocating molecules
Size limit to rapid entry:
prevents entry of larger molecules
e.g labelled dextrans of various sizes
allows passive diffusion of small molecules
Therefore effective pore diameter is…
9nm
effective pore diamter vs channel of the pore complex
effective pore diameter (9nm) is much smaller than the 60nm channel of the pore complex
effective pore diamtere is controlled by the physical properties of the hydrogel
→ TOO SMALL for proteins larger than 40-60 kDa
If it is too small for proteins larger than 40-60kDa, than we have a problem….
how do large RNA molecules and large proteins interact with the hydrogel and cross NPC
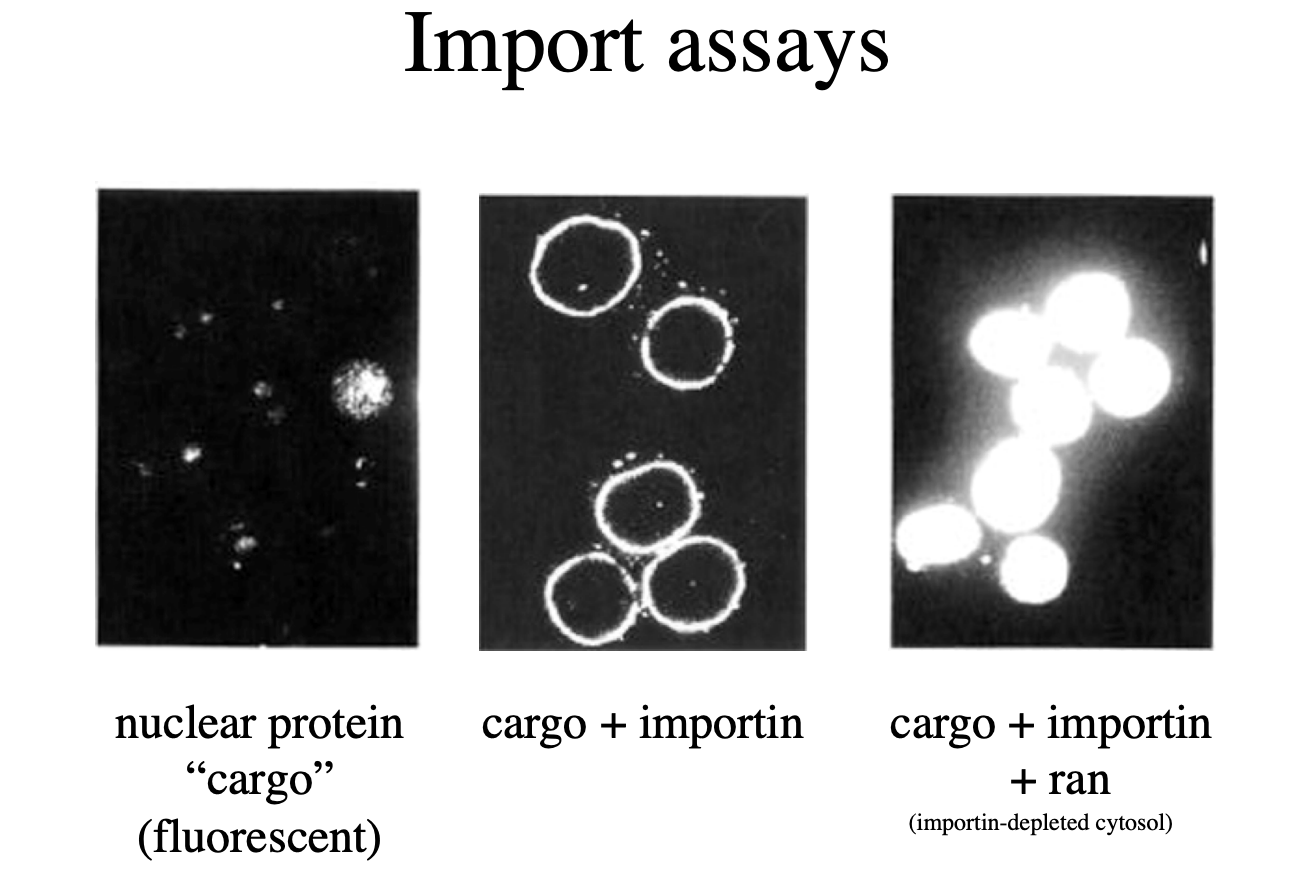
Assays to study import
Using frog oocytes
pentamer protein from a frog with radioactive nucleoplasmin microinjected→ allowe in
Need to check what is actually causing the importation→ take off the radioactive tails→ no uptake
Just microinject the tails→ UPTAKE
but is this actually through the pores??
Add tails to colloidal gold particle→ (attached to something that isn’t a protein
RESULT: there is uptake into nucleus through nuclear pores
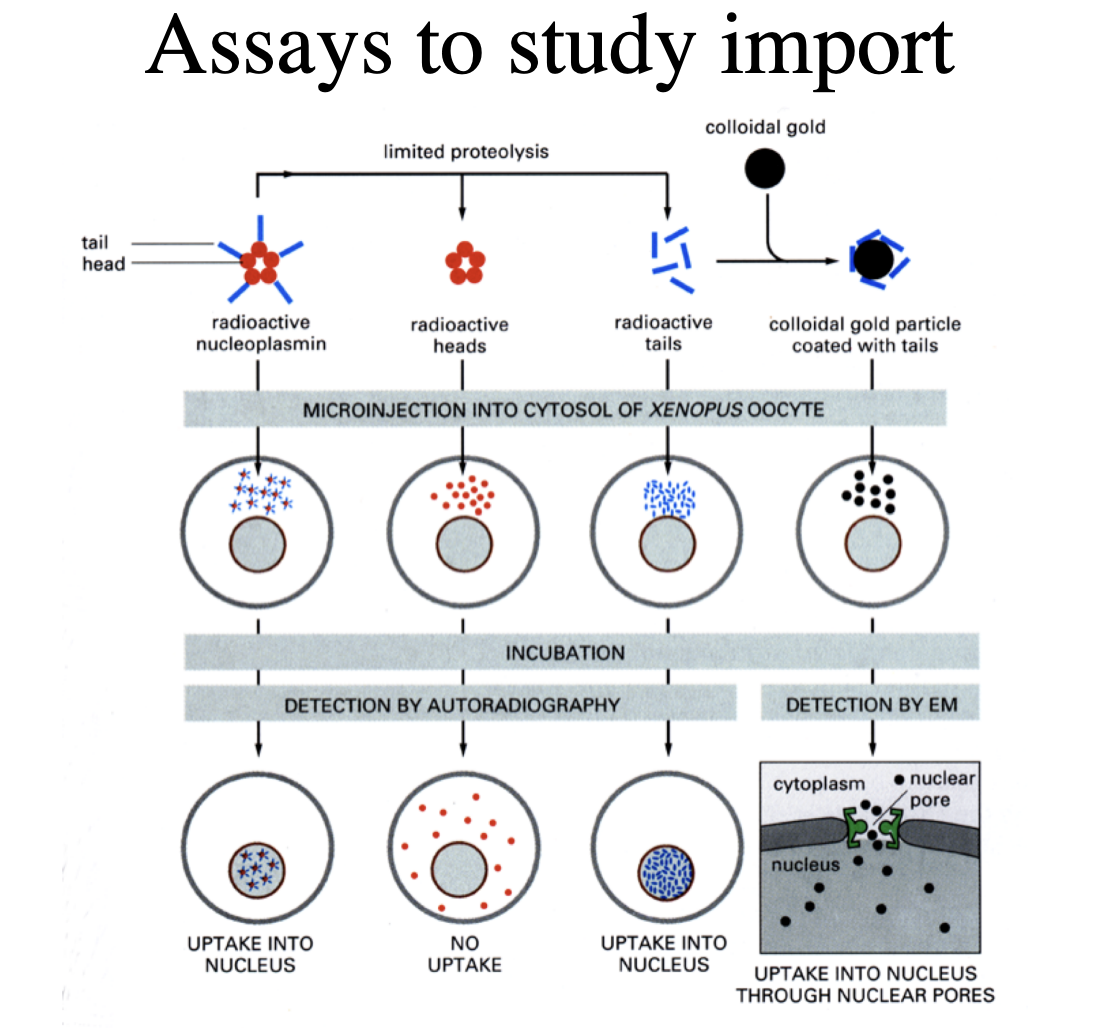
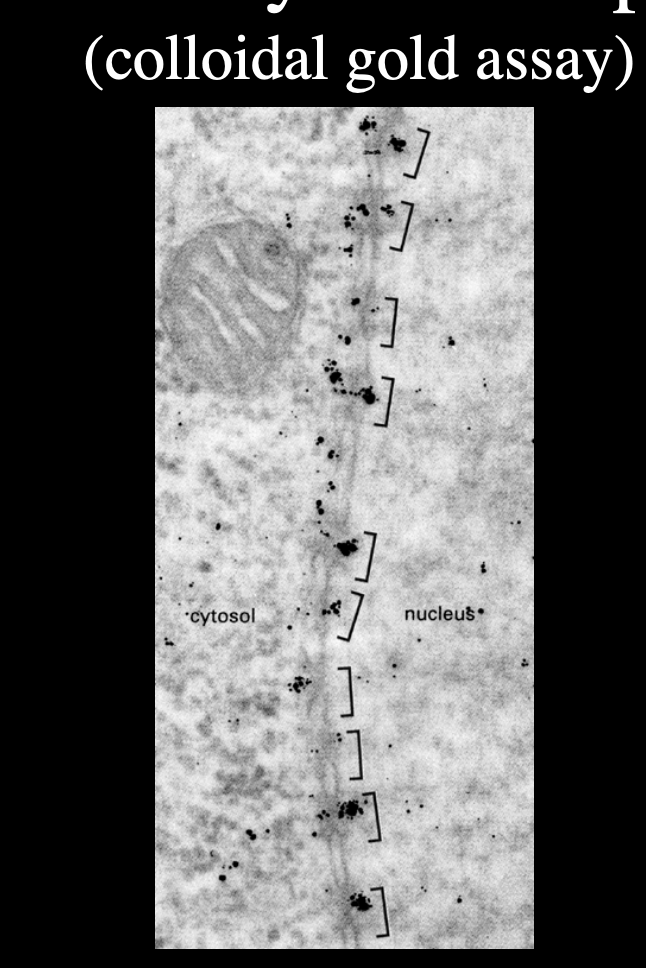
Tail coated gold particles
Gold particles all line up and enter through nuclear pore
→ gold is too big a particle→ so highlights where the pores are
Import of nuclear proteins: what proteins can accumulate in the nucleus
proteins of all sizes can accumulate in the nucleus by >100-fold
Import of nuclear proteins: in microinjection experiments into the cytoplasm
nuclear proteins re-accumulate in the nucleus
how is this acheived:
selective entry through the nuclear pore complexes
Import of nuclear proteins: underlying molecular mechanism has been eluciated by…
Studying the import of the nuclear proteins:
nucleoplasmin
SV40 large T antigen
Nuclear localisation signals (NLS)
proteins localisaing to the nucleus
e.g nucleoplasmin and SV40 large T antigen
contain small peptide motifs that cause their import
when fused to other non-nuclear proteins (E.g BSA)→ cause nuclear accumulation of the fusion protein
bind to specific carrier proteins terms importins (karyopherin in yeast)
highly conserves and can be transferred into other things→ causes ther things to be transported across
But can be species specific→ e.g bird flue cannot be transported as well across the human nucleus as human flu can be
Forms INTEGRAL part of the protein

Why important to have an NLS
ensures that it is recognised for import VIA proteins
ensure that the whole protein doesn’t have to be a specific property etc to get through the hydogel
instead→ it is the import that does the work and so the protein only needs a TINY NLS to allow it to be able to get through the nucleus
→ otherwise→ only a limited range of protteins would be able to get through the gel
NLS modifications
Forms an integral part of the protein
BUT
can be masked → so can stop/start its import
Can also have BOTH NLS and Nucleuar Export signals
ca change masking on each to swtich on and off (in/out)
What do mutations of these proteins cause
mutation to lysines of nucleoplasmin
mutation of threonine or asparagine SV40 T antigen
→ abolish transport
The bold amino acids of the respective NLS were found to be essential”

What did injection of colloidal gold coated with nucleoplasmin into the cytoplasm reveal
passage through the central channel of the NPC
Nuclear transport: in two steps
rapid binding of the cargo to the cytoplasmic side of the nuclear pores
then, slower energy-dependent translocation through pores
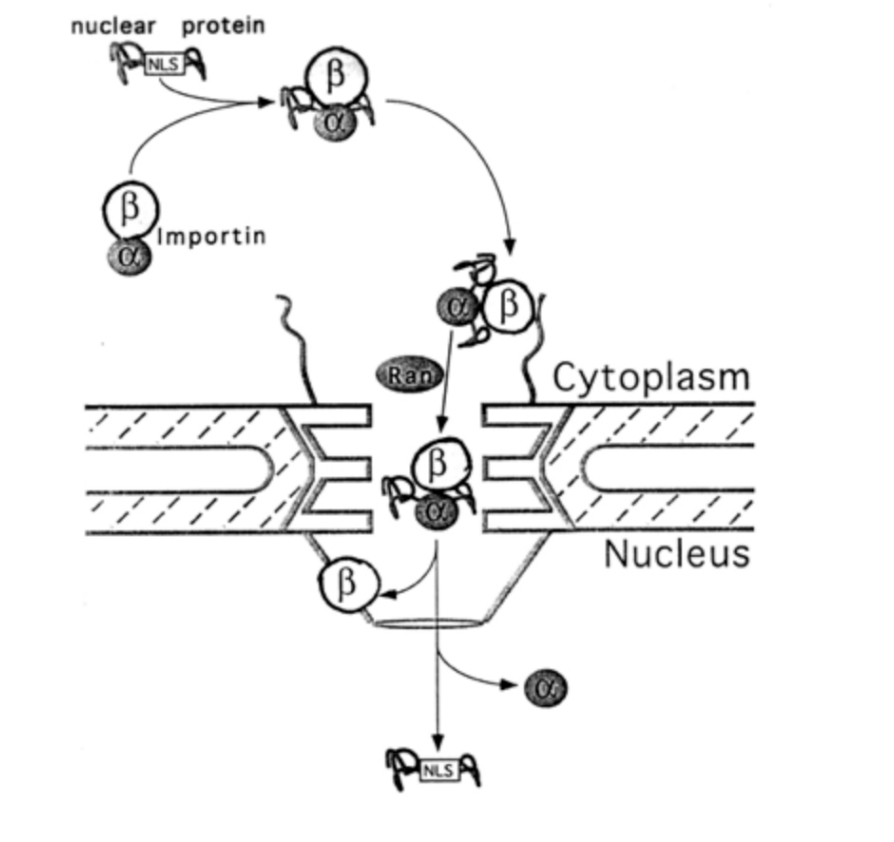
What is required for these two steps
soluble key proteins:
Importin
Ran→ a small GTP-ase
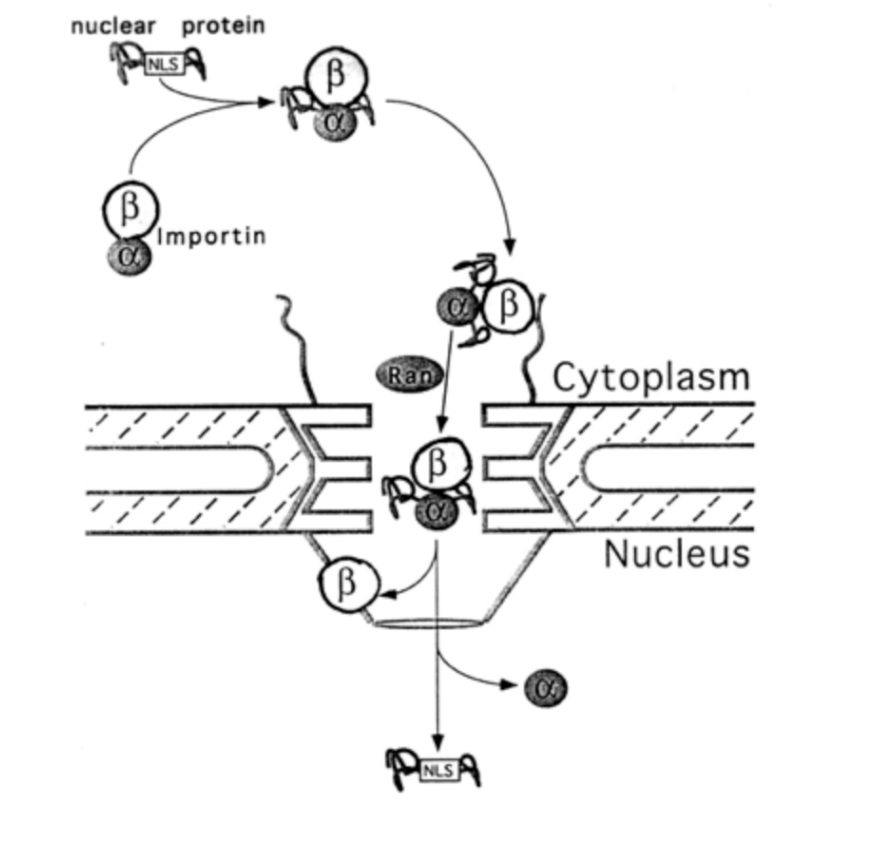
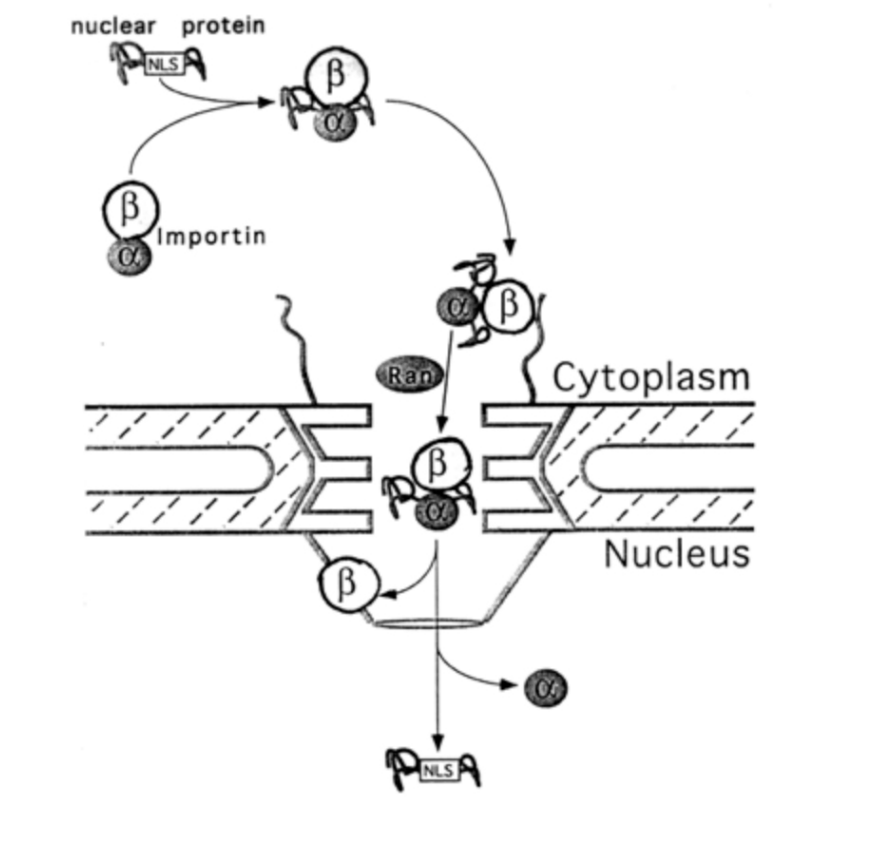
What is the process with these proteins
alpha subunit of importin binds the NLS of the cargo
the beta subunit docks at the nuclear pore complex
importins have spots that are hydrophobic and stick to the hydrophobic parts of the net
allows the net to open up as it moves in
but close again as it moves through
following transport through the NPC, nuclear ran-GTP causes dissociation of importin ffrom NLS-cargo
Importin alpha and beta are separately exported back into the cytoplasm in a ran-GTP dependent manner
importin beta reversibly interacts with FG repeats of the NPC
Evidence for the importance of all three features
Nuclear localisation signal
importin
ran
Roles of importin subunits
Alpha→ binding to the NLS of cargo
import receptor for many cargoes
binds to importin-beta
Beta→ binding to NPC and ran
interacts with FG repeats
is the actual import mediator
bigger than alpha
can bind to hydrophobic interaactions (see previously)

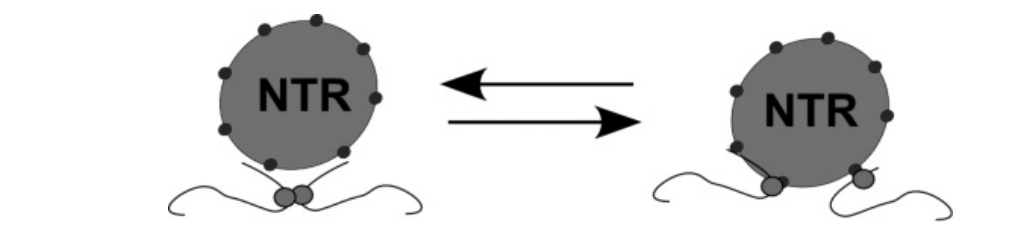
Importin beta reversibly interacts with FG repeats of the NPC → shows the hydrophobic interaction between the blobs and the beta subunits
THEREFORE: it can cross the hydrogel and act as import mediator through the gate
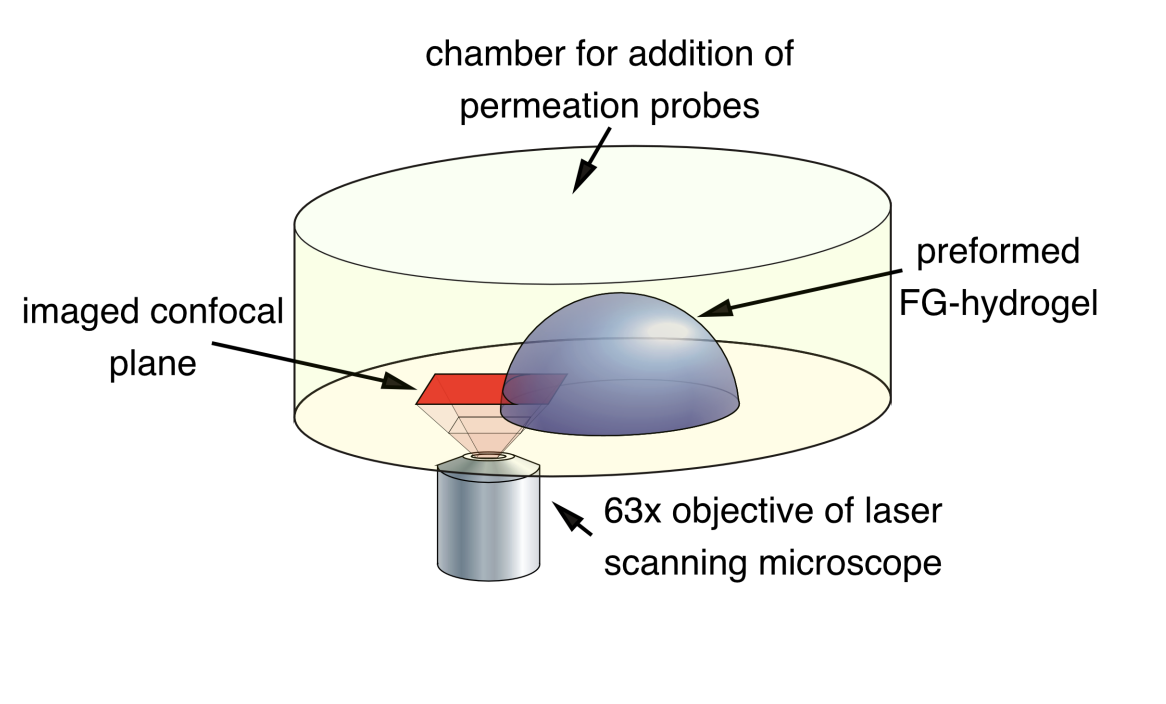
Experimental evidence for diffusion of a transport receptor into hydrogel (seen above: experiment set up
chamber with buffer
uses phase contrast and fluorescent microscopy
Measures the diffusion into FG-hydrogel of:
A-IBB-RedStar (140kDa)→ importin beta binding domain of importin alpha
B-IBB-Redstar in complex with importin beta (530 kDa)
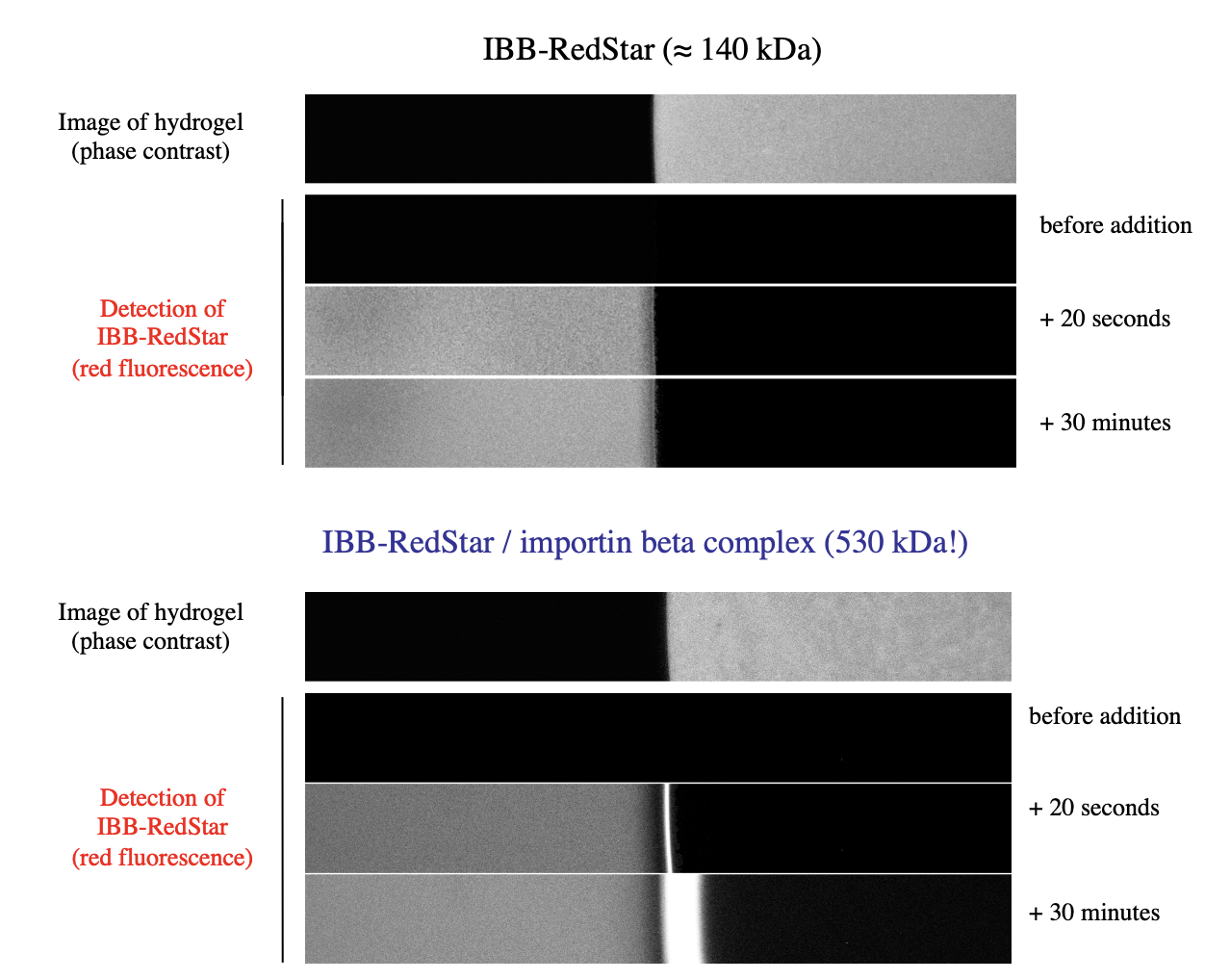
Experimental evidence for diffusion of a transport receptor into hydrogel (seen above: results
Left: buffer
Right: hydrogel
with IBB-RedStar→ NO protein transfer
with IBB-RedStar/ WITH importin beta complex→ HAD SOME IMPORTED protein
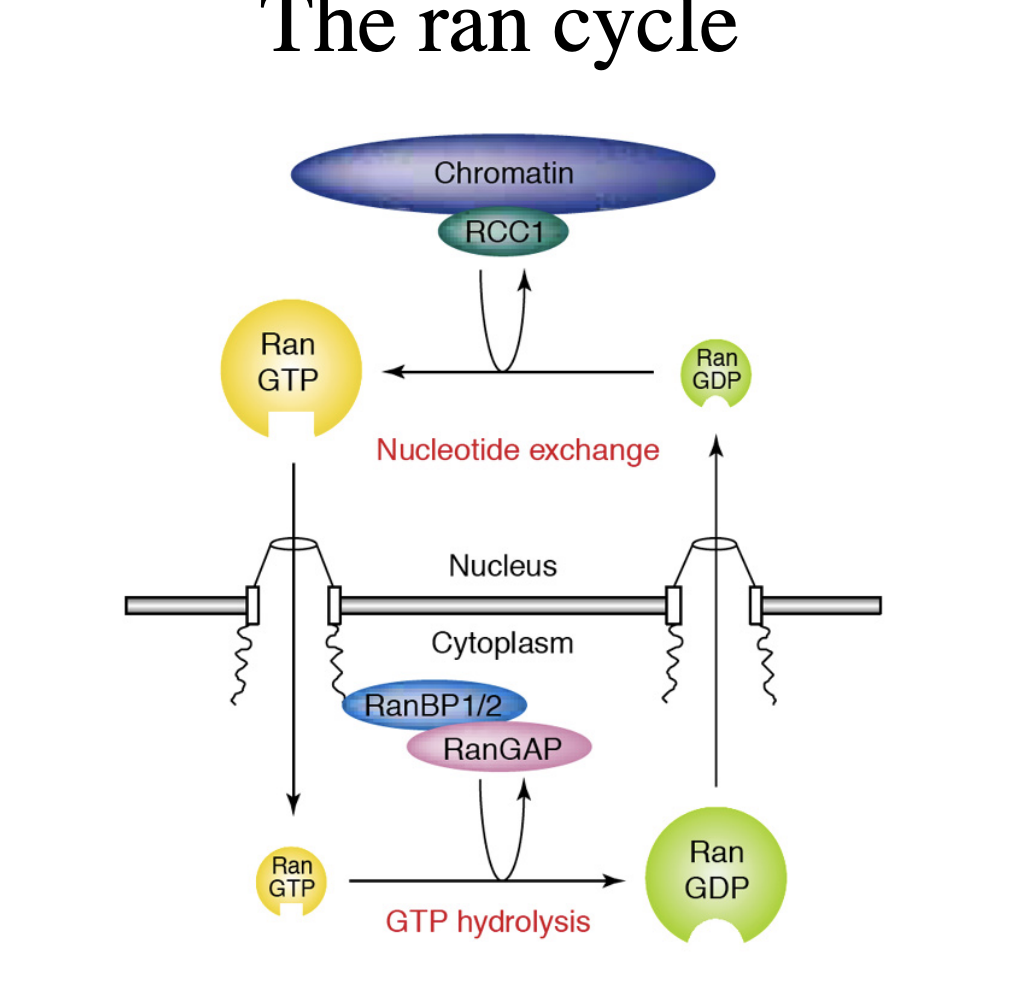
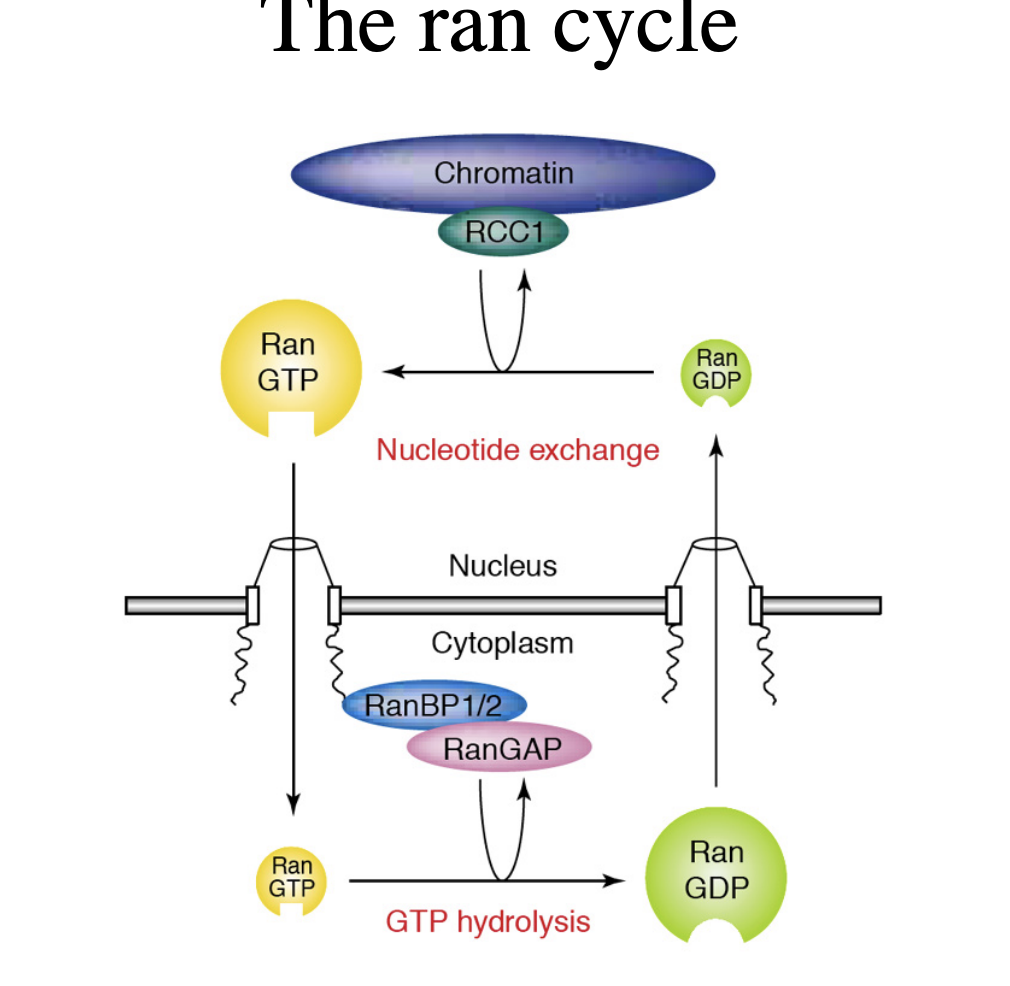
The ran cycle: small GTP’ase ran is involved in the nuclear import (and export)
ran can bind GDT or GTP
Ran GDP is predominantly cytosolic
ran GTP is predominantly nuclear
in the cyotsol, ran-sepficic GAP (GTPase activating protein) stimulates the endogenous GTPase of ran
e.g RanBP1/2 and RanGAP
converts it to the GDP form
in the nucleus, chromatin-bound nucleotide exchnage factor RCC1 promotes exchange of the bound GDT to GTP (Ran GAF?)
RCC1 also regulates chromatin condensation
thus building a ran-GDP/ran-GTP gradient across the nuclear envelope
Ran GDP vs Ran GTP
Ran GDP→ cytosolic
required for cargo binding to importins in the cytosol
Ran GTP→ nuclear
promotes cargo dissociation from importins in the nucleus
conversely, ran GTP is involved in cargo binding to exportins in the nucleus
Asymmetric distribution
how to remember→ C is before N in the alphabet and D is before T in the alpha bet→ therefore cytosol has more Ran GDP
Regulated import: what is required for the regulation of nuclear import of NLS-containing cargo
ran cycle be coupled to the importin cycle
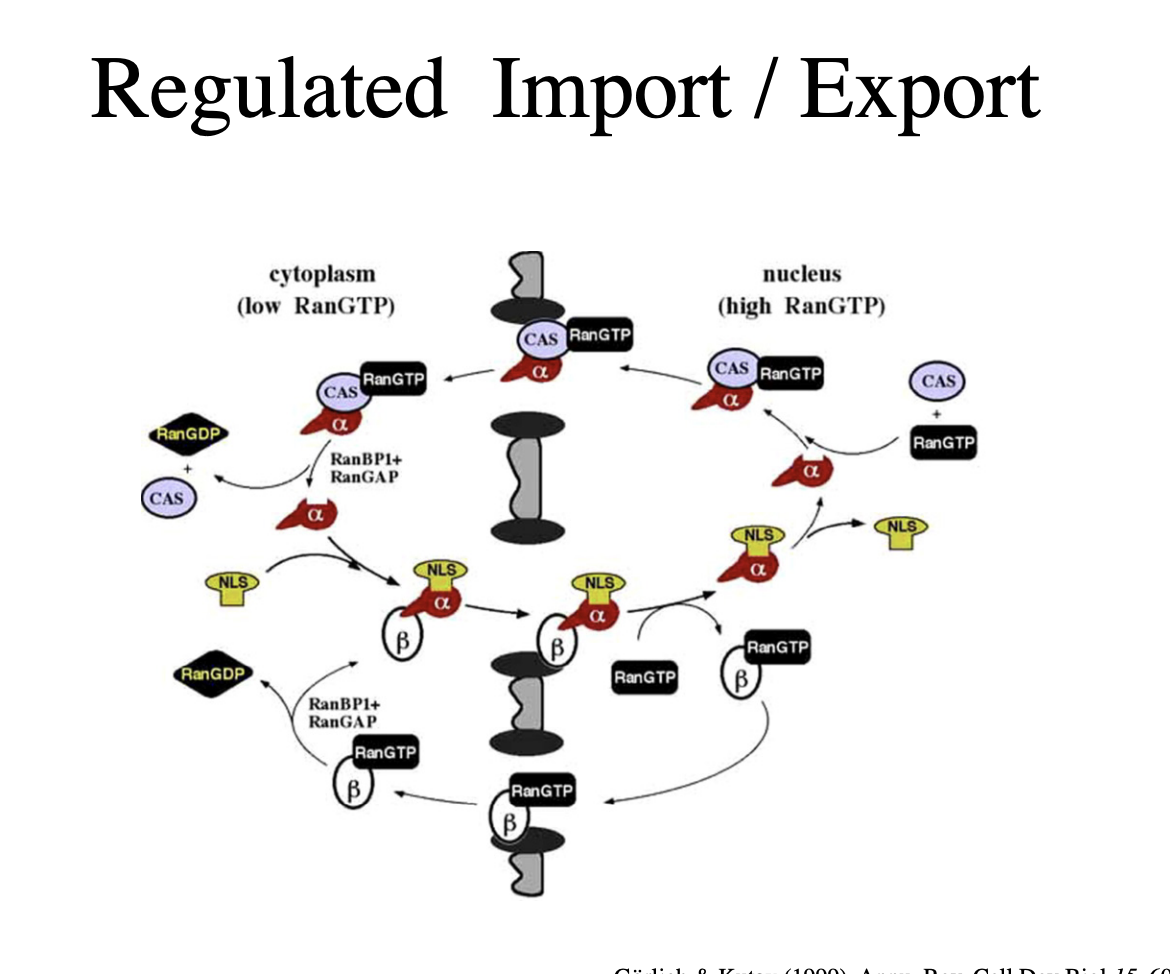
Summary of the regulated import/export
note: with the import, there is then export of the proteins that help this import in→forms an equilibirium of masses in and out
NLS recognised by alpha subunit of importin and binds
beta subunit then binds using GTP
interact and bind to the pore
moves it through the pore
beta subunit has higher affinity for RanGTP which high in the nucelus
beta subunit dissociate off and is stable with the RanGTP
alpha subunit then off laods
Next the alpha and beta subunits both are exported back into cytoplasm for further import:
alpha subunit:
CAS binds and then ranGTP binds
goes through pore
in cytosol, RanGTP-CAS is unstable and dissociates off with hydrolysis
form Cas + Ran GDP
beta subunit
stable as beta-RanGTP
moves through pore
now unstable and ranGTP hydrolsyses off to RanGDP
beta subunit now free for further cargo
note: all of the hydrolysis and removal of RanGTP from subunits also uses the RanBP1 and RanGAP for activation (seen above)
Export of proteins and RNA
RNA is exported as protein complexes
signal dependent and carrier mediated
occurs through the nuclear pore complex
What is the process of this export of proteins and RNA
giant mRNA transcripts from insect salivary glands (35-40kb) unfold to pass through the pore
always 5’ first
before mRNA is exported→ must be processed correctly
including splicing and poly A addition
Nuclear export signals (NES)
have been recently been identified in several exported proteins
including some that bind RNA

How is export regulated
export signals are recognised by export receptors (exportins) that are regulated to importin beta
the regulation of cargo export again depends on the ranGTP/ranGDP gradient across the nuclear envelope
ran protein levels are equilibrated across the evelope by the nuclear transport factor NTF2
ensures there is enough on either side for import/export
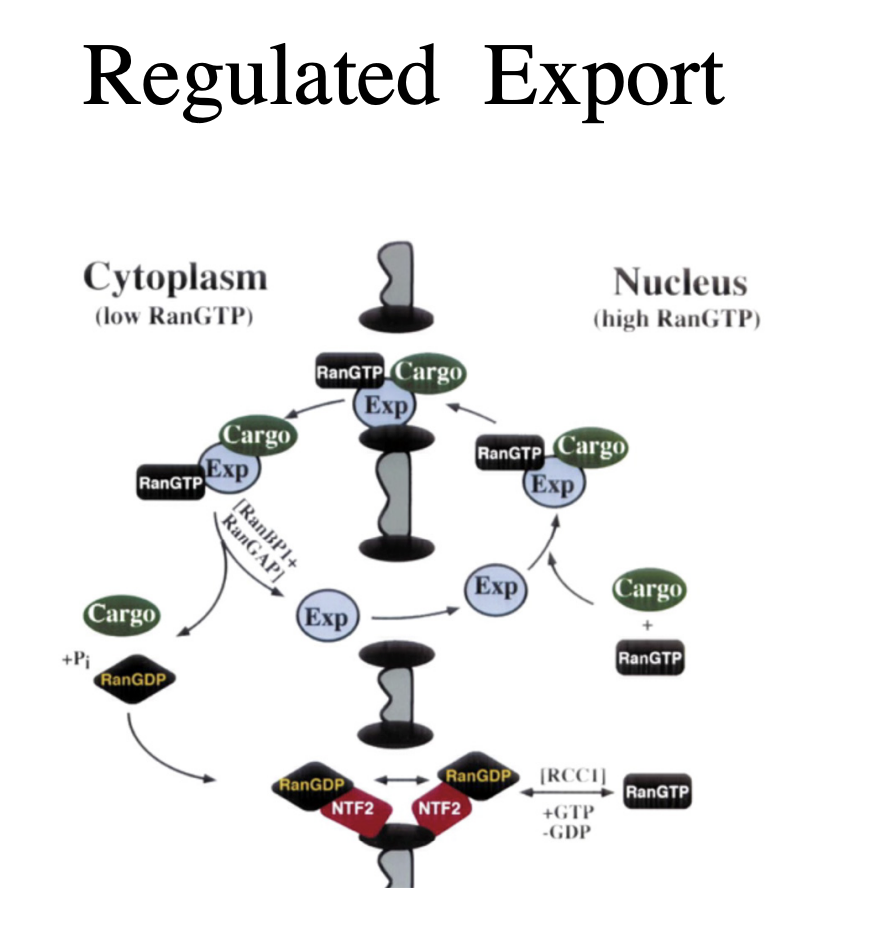
Process of export regulation
HV unspliced transcipts bind to the Rev protein
this mediates export via interaction with an exportin
Small RNAs bind their own exportins directly
tRNAs interact with exportin-t
other small non-coding RNAs such as microRNA precursosrs interact with exportin 5
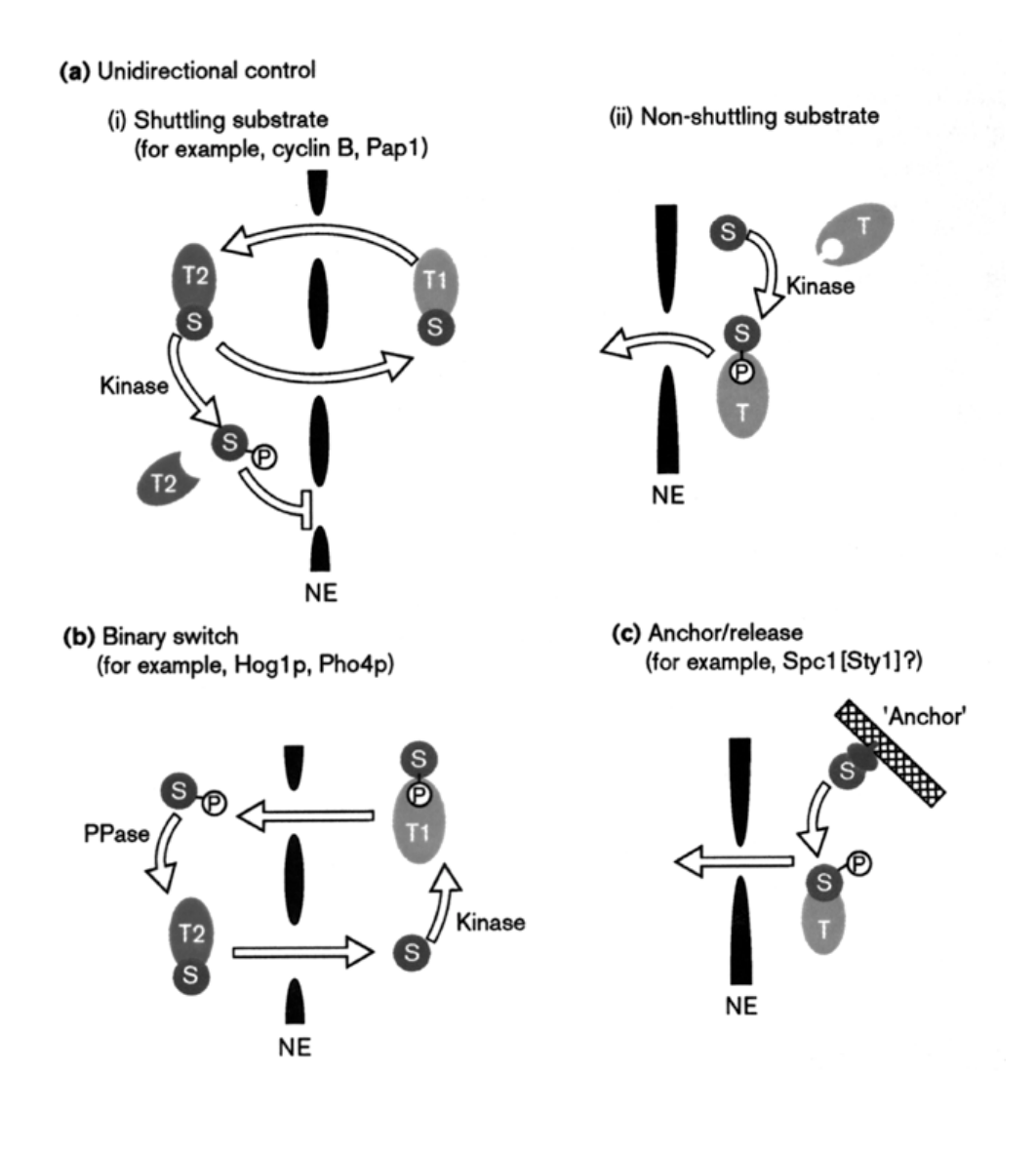
Higher levels of regulated transport (ensure import/export only happen when you want it to): 1 some proteins…
can only enter the nucleus when released from cytoplasmic anchors
e.g: several transciption factors
cytoskeleton
plasma membrane?
→ this provides a possible level of gene regulation
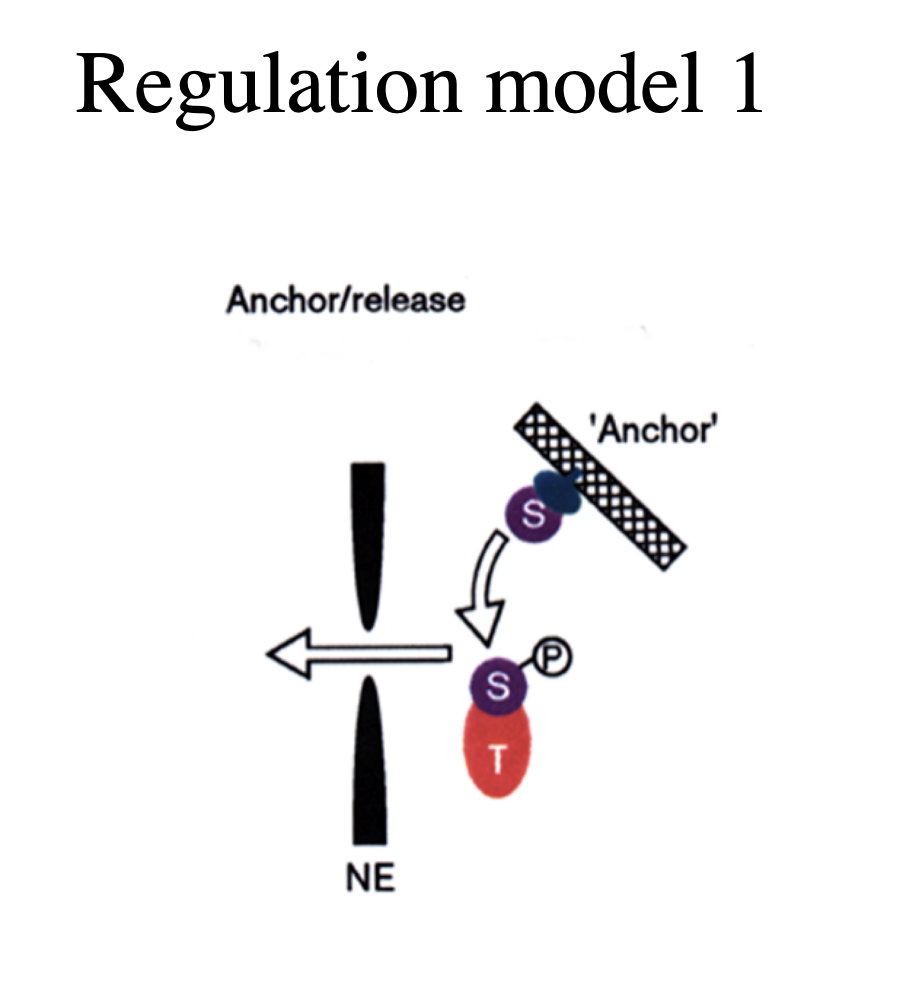
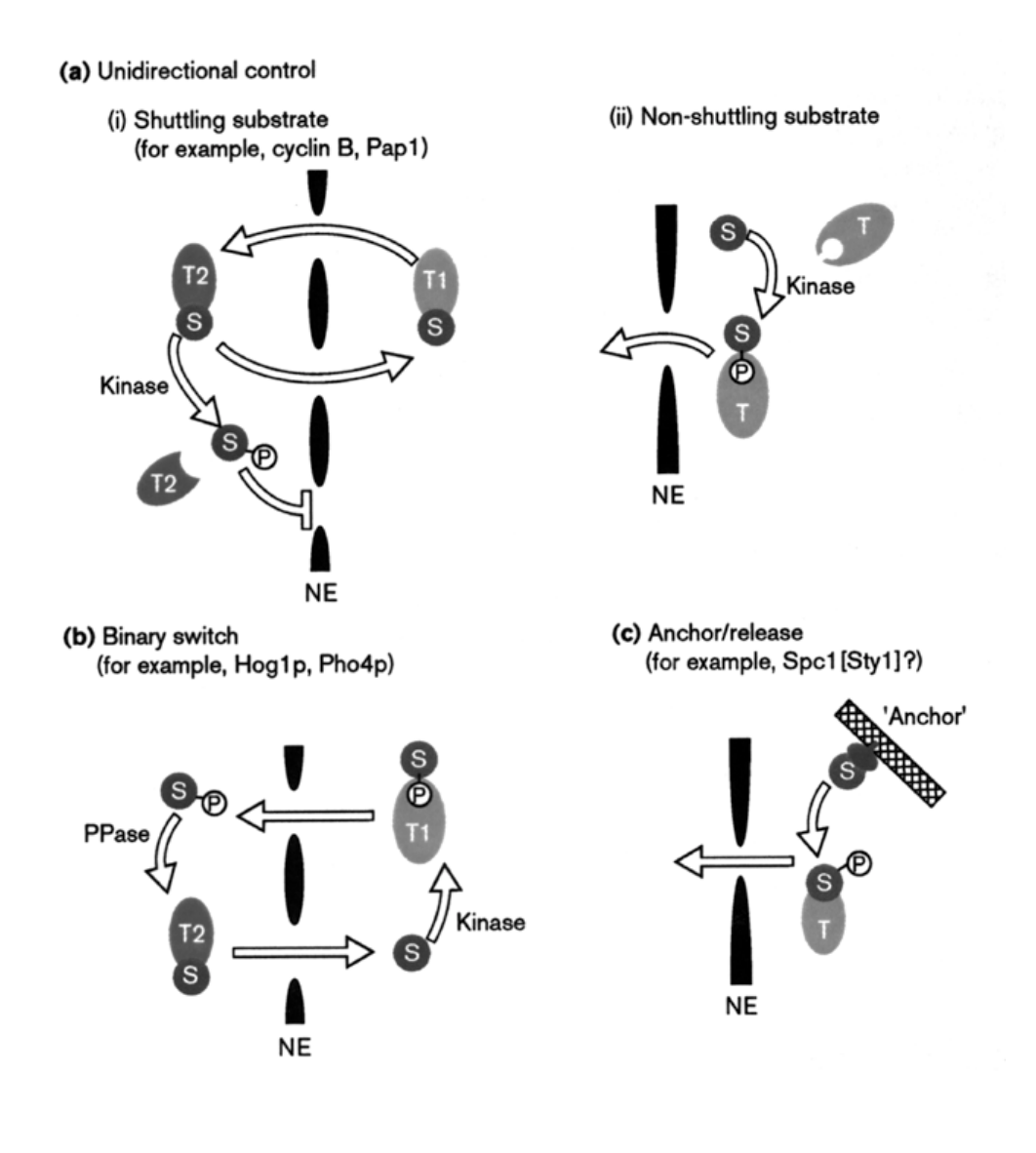
Higher levels of regulated transport: 2 Phosphorylation of NLS or NES eleents
another discussed means to regulate nuclear transport:
e.g:
cyclin B1 constantly shuttles between nucleus and cytoplasm in S and G2 phase
but phosphorylation of NES is early mitosis BLOCKS export
→ results in nuclear accumulation
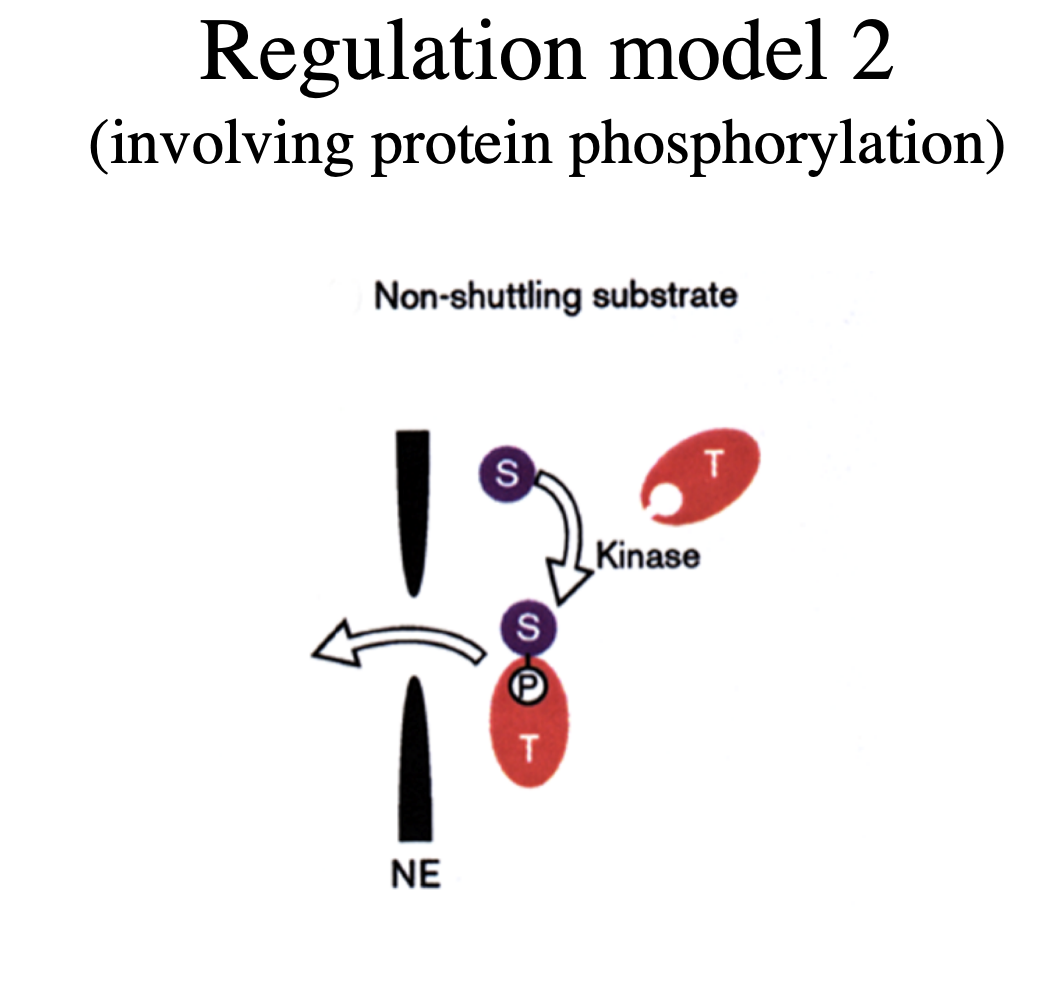
See again: regulation model 2 (involving protein phsophrylation)
Regulation model 2b (involving protein phosphorylation)
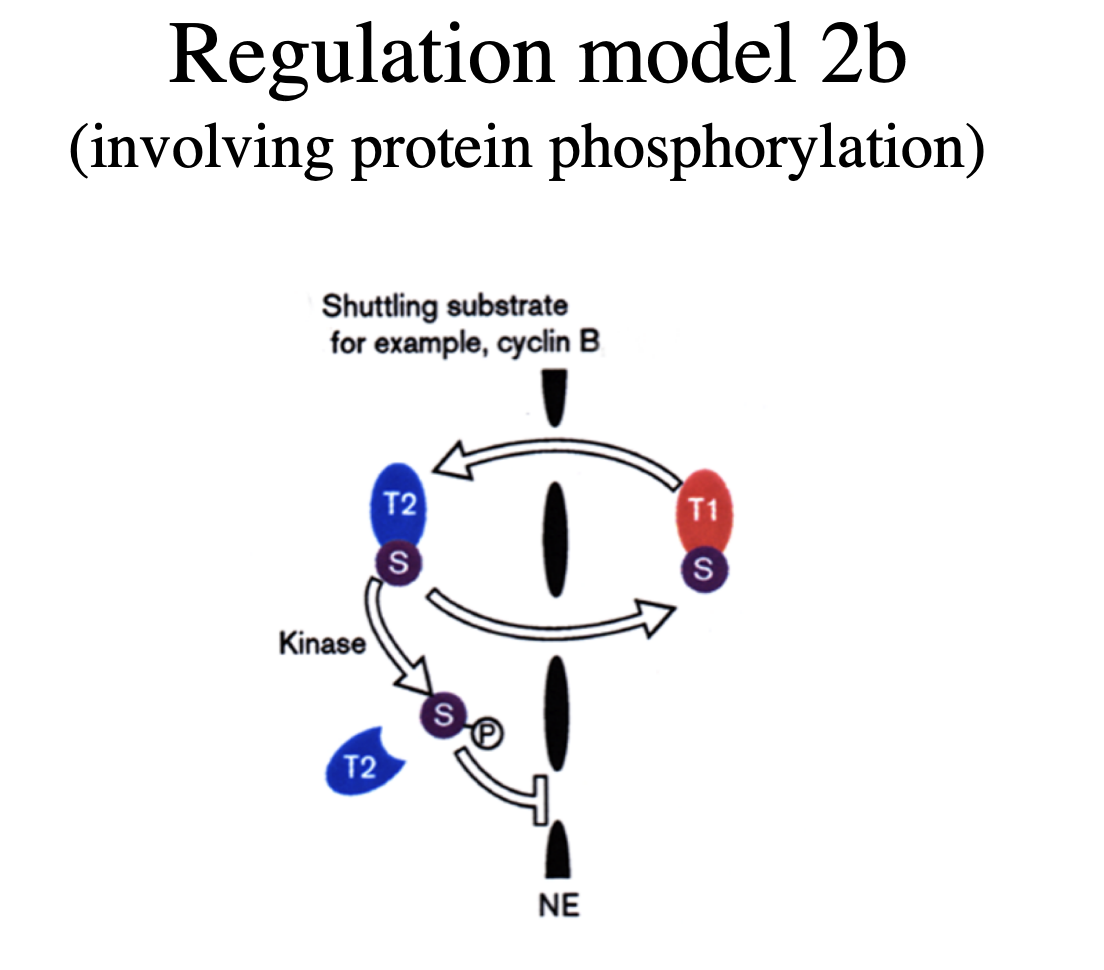
Regulation model binary switch
when the protein has BOTH NLS and NES
can be masked on and off
or may be both on
Regulates the ferrying between nucleus and cytosplasm