Energy changes
1/39
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
40 Terms
Endothermic or exothermic: energy is transferred to the surroundings
Exothermic
Endothermic or exothermic: the temperature of the surroundings increases
Exothermic
Exothermic or endothermic: combustion, oxidation and most neutralisation reactions
Exothermic
Exothermic or endothermic: self-heating cans and hand warmers
Exothermic
Exothermic or endothermic: energy is taken from the surroundings
Endothermic
Exothermic or endothermic: the temperature of the surroundings decreases
Endothermic
Exothermic or endothermic: thermal decomposition reactions and the reaction of citric acid and sodium hydrocarbonate
Endothermic
Exothermic or endothermic: instant ice packs
Endothermic
Energy is _____ when bonds are broken or bonds are formed
Transferred
During a chemical reaction, bonds in the ____ are broken and new bonds are made in the _____ (give answer in the form ____, ___)
Reactants, products
Exothermic or endothermic: more heat energy is released in making bonds than is taken in when breaking bonds in the reactants
Exothermic
Exothermic or endothermic: less heat energy is released in making bonds than is taken in when breaking bonds
Endothermic
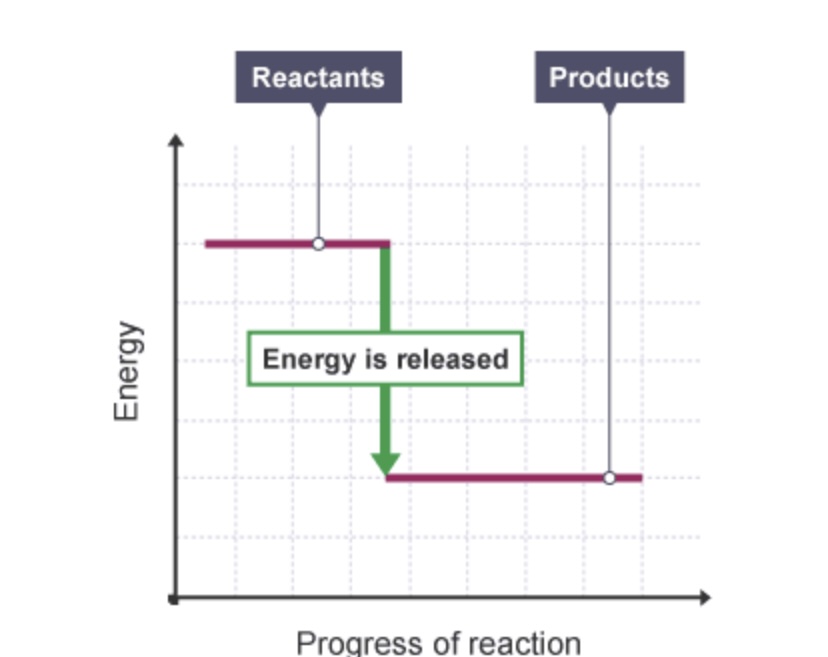
Does the energy level diagram show and exothermic or an endothermic reaction?
Exothermic
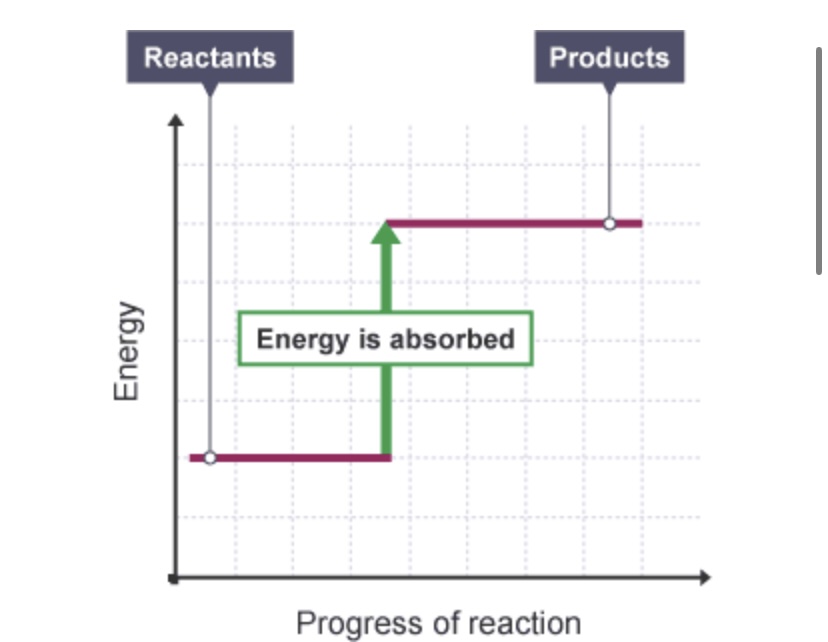
Does the energy level diagram show an exothermic or an endothermic reaction?
Endothermic
What is defined as ‘the minimum energy needed by particles when the collide, for a reaction to occur’
The activation energy
In the profile for an exothermic reaction, the overall energy change is _____
Negative
In the profile for an endothermic reaction, the overall energy change is _____
Positive
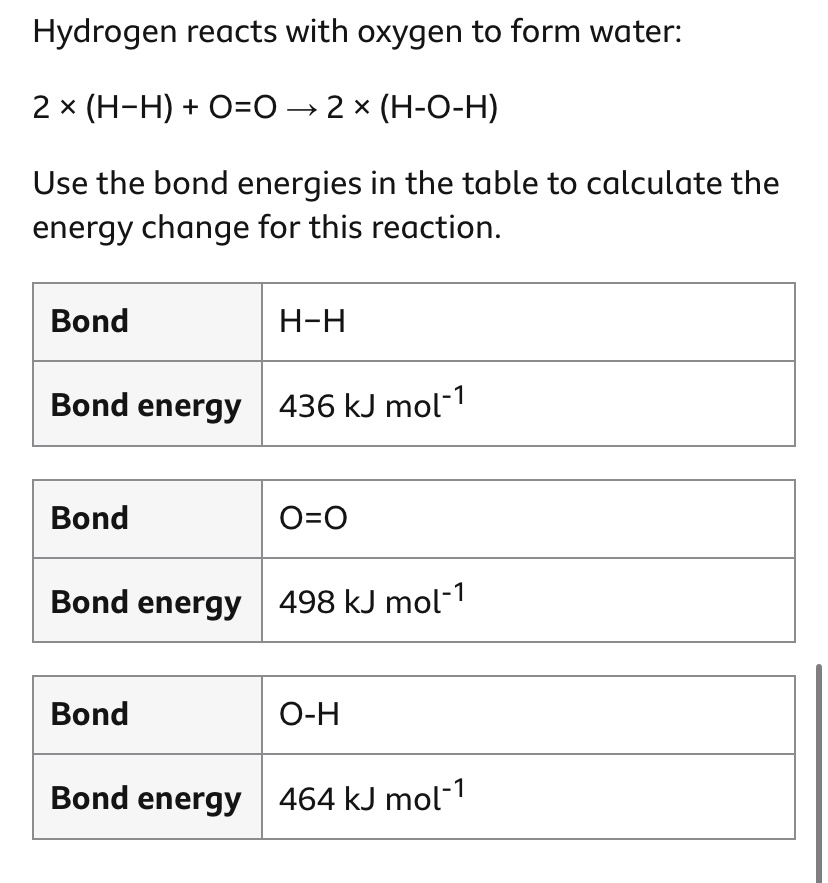
The answer is _____ KJmol to the power of -1
-486
Chemical cells use ____ ____ to transfer energy by electricity
Chemical reactions
Connecting two different metals in contact with an electrolyte makes a ____ _____
Simple cell
What is one example of a non-rechargeable cell?
An alkaline cell
In a non-rechargeable cell, a voltage is produced until until one of the reactants is used up and the battery ____ ____
Goes flat
In chemical cells, swapping the two electrodes means that the recorded voltage becomes _____
Negative
The biggest voltage in a chemical cell occurs when the difference in the reactivity of the two metals is the _____
Largest
Which will have a higher voltage, a chemical cell made from magnesium and copper or a cell made from magnesium and silver?
A cell made from magnesium and silver
What type of cell will continuously produce a voltage as long as it has a constant supply of a suitable fuel and oxygen?
A fuel cell
In a fuel cell, the fuel is oxidised _____ rather than being burned so reaction can take place at a lower temperature
Electrochemically
In a fuel cell, the energy is released as _____ energy not thermal energy
Electrical
What is a good alternative to rechargeable cells and batteries?
Hydrogen-oxygen fuel cells
What is the only product formed in a hydrogen-oxygen fuel cell?
Water
Which type of cell is cheapest to manufacture?
An alkaline cell
Which type of cell may end up in landfill or is very expensive to recycle?
An alkaline cell
Which type of cell can be charged many times, limiting the amount of resources used?
A rechargeable cell
Which type of cell is quite expensive to manufacture?
A rechargeable cell
Which type of cell is easy to maintain as there are no moving parts?
A hydrogen fuel cell
Which type of cell is small in size?
A hydrogen fuel cell
Which type of cell has water as its only product?
A hydrogen fuel cell
Which type of cell is very expensive to manufacture?
A hydrogen fuel cell
Which type of cell needs a supply of a flammable gas?
A hydrogen fuel cell
Which type of cell is used in spacecraft?
A hydrogen fuel cell