registry 2025 - target and matter interactions
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
Bremsstrahlung
this interaction:
- happens in the tube
- incoming negative electron passes outer shell and gets close to positive nucleus
- slows down in trajectory and curves from the nucleus
- that slow down in kinetic energy causes a release of high energy xray photon
- most of the x-rays are from this type of interaction
- and each time that slowed down electron makes runs with other nucleus, that resultant photon has less and less energy
- is 70% to 90% of the xray beam
hetergeneous beam
- This is a quality in brems radiation
- also called polyenergetic
- called this because you have a variety of energy in the beam from high to low
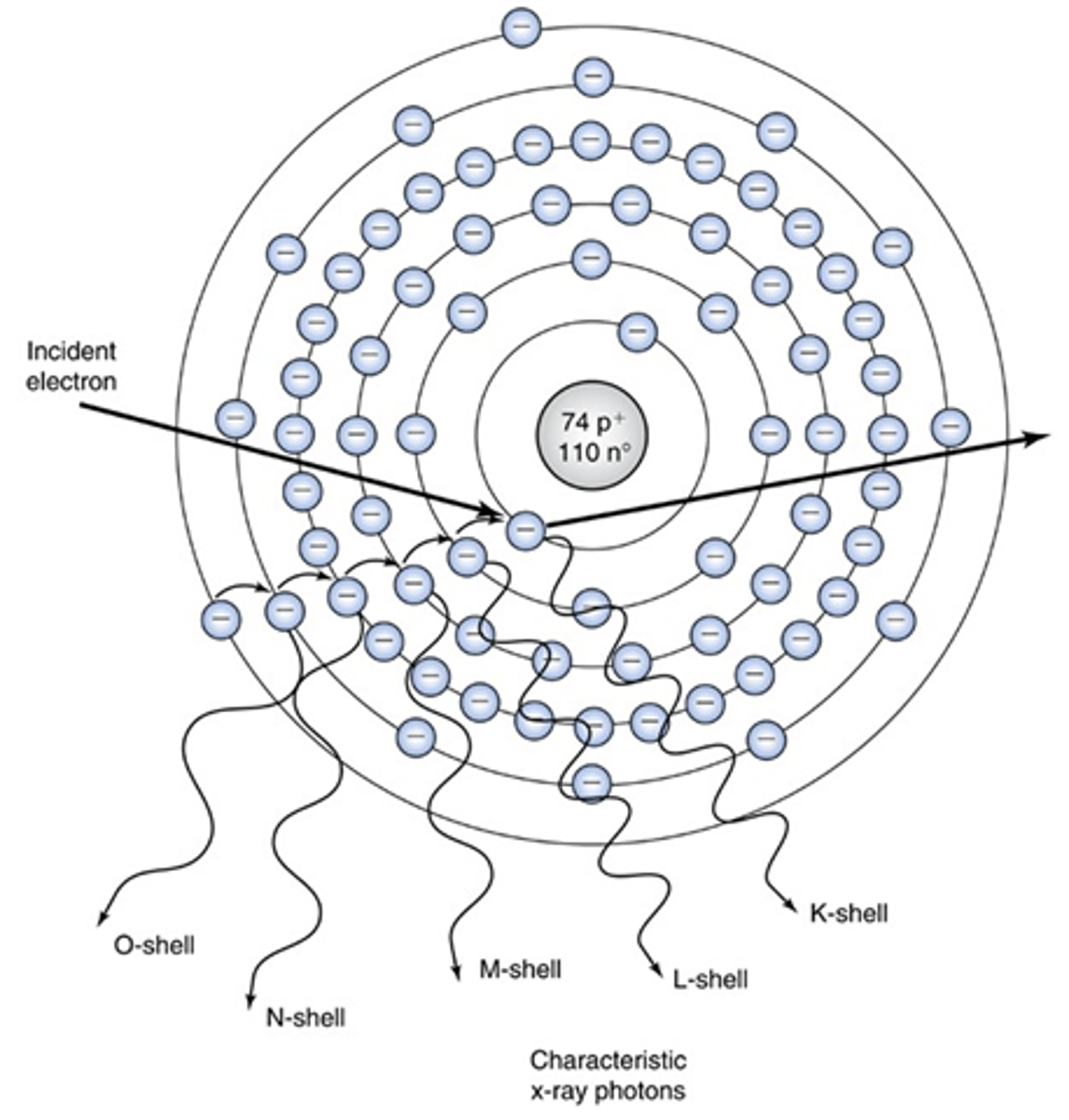
characteristic radiation
this interaction:
- A projectile electron collides with an inner shell electron of a target atom and gets ejected from orbit and
ionizes the atom
- A hole exists in the inner shell from the vacated electron and an electron from an outer shell falls in to fill the hole
- Then when the electron falls in, energy is given off in the
form of an x-ray photon
- This creates a hole in its shell of origin, and an electron from the next outer shell falls in to fill this vacancy; this continues until the atom is stable again
- Each time an electron falls in to fill a hole, an x-ray
photon is given off and each x-ray photon has a specific energy, equal to the difference in the binding energies of the two shells involved
- Only x-rays produced at the K-shell are of sufficient energy to be used in diagnostic radiography
- These x-rays possess energy that's typical of the specific binding energies of the atom involved.
- these x-rays are produced at kVp levels greater than 70 but only in small numbers
- makes up 10% to 30% of the x-ray beam
discrete x-ray spectrum
This is a typical for characteristic radiation.
This is called this because energies involved are specific to the
target atom and are predictable.
Continuous x-ray spectrum
Typical of brems radiation
because these energies all are different (from the peak electron energy down to zero energy)
photoelectric
This interaction:
- is considered characteristic as the incoming x-ray photon hits the k-shell electron. That electron gets ejected.
- the photon becomes COMPLETLEY ABSORBED and ceases to exist
- occurs in tissues
- results in increased dose to the patient
- k-shell hole gets fills by the other electrons in the other shells releasing low energy photons.
- this produces contrast in the radiograph because of the differential absorption of the incoming x-ray photons in the tissues
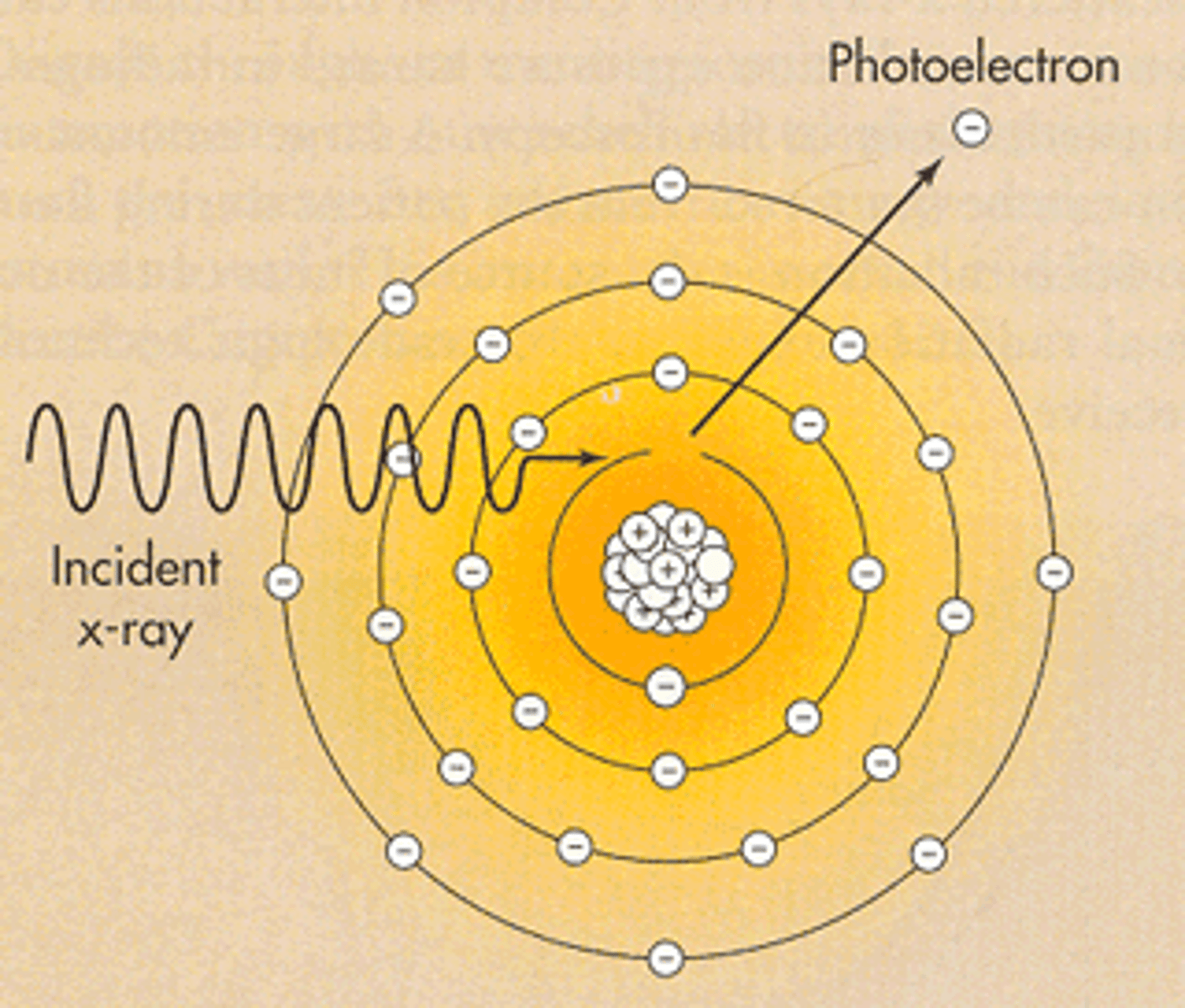
Photoelectron
this happens in photoelectric interactions. Its the electron that got ejected from the k-shell.
it may get ionized or excite other atom until it deposits all its energy
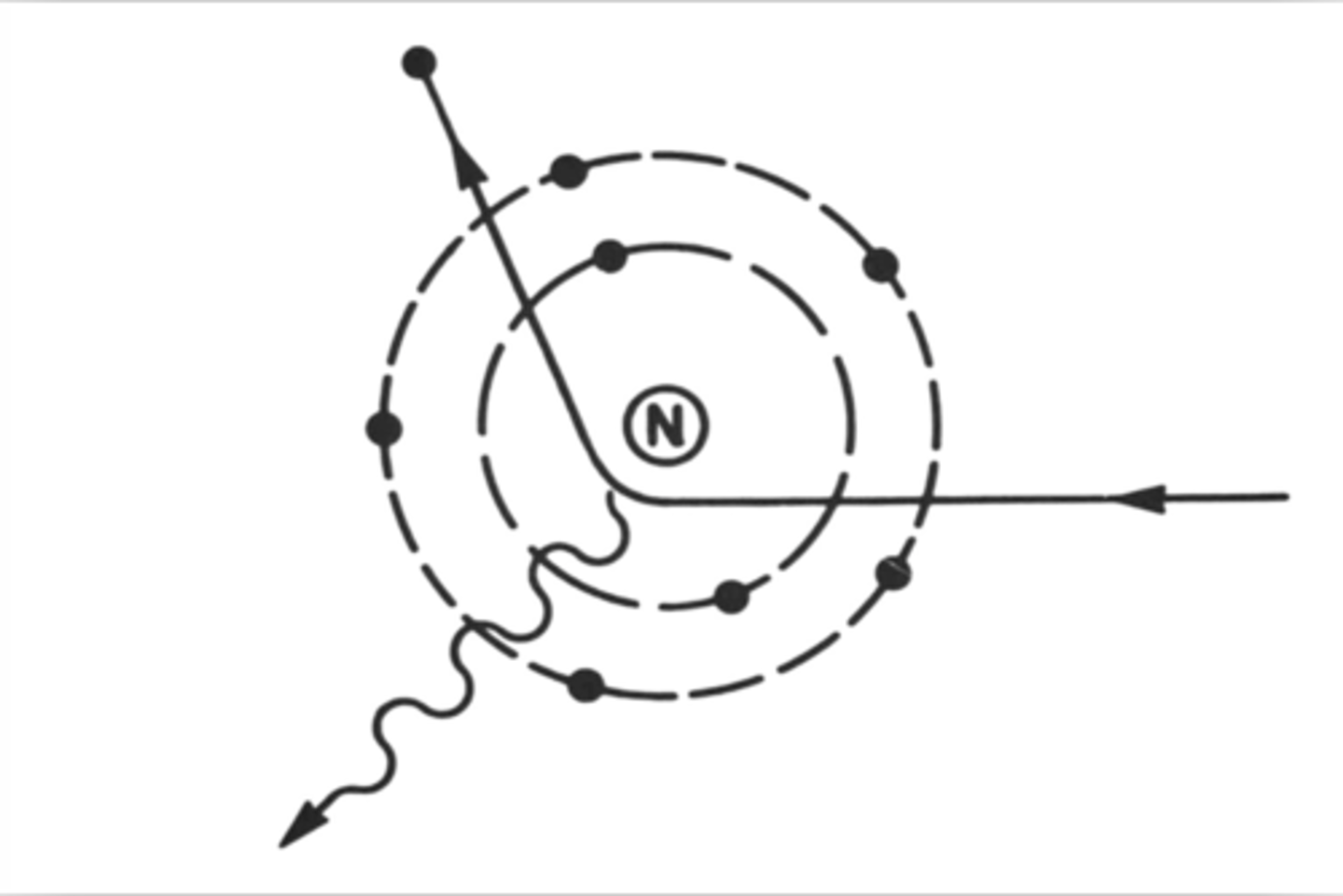
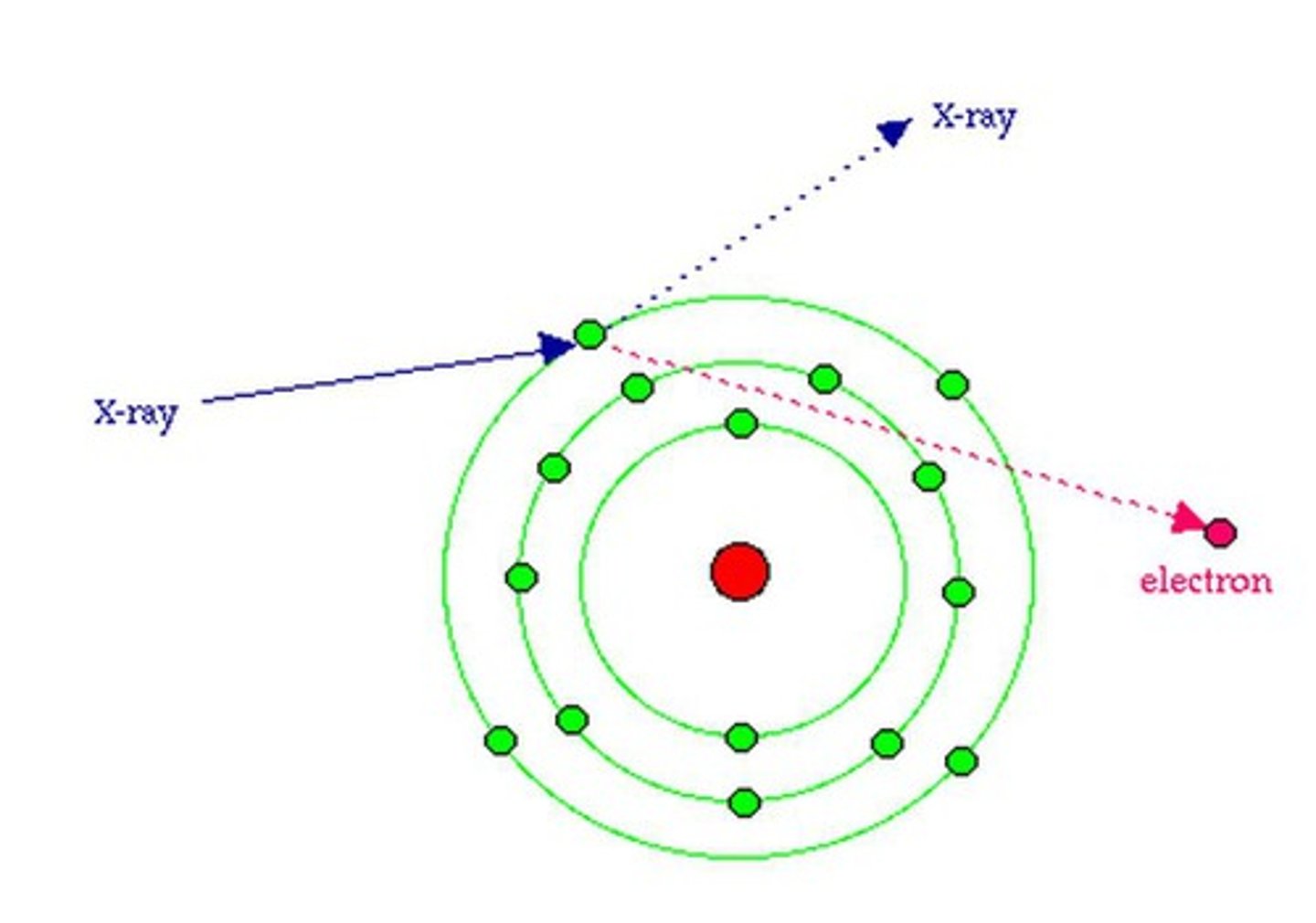
Compton scatter (interaction)
- occurs in tissues
- also called modified scattering
- Incoming x-ray photon strikes a loosely bound, outer-shell electron
- Photon transfers PART of its energy to the electron
- Electron is removed from orbit as a scattered electron, referred to as a recoil electron
- Ejected electrons may ionize other atoms or recombine with an ion needing an electron
- Photon scatters in another direction with less energy than before because of its encounter with the electron
- Scattered photon may interact with other electrons, causing more ionization, additional scattering events, or photoelectric absorption; or it may exit the patient
- Scattered photons emerging from the patient travel in divergent paths in random directions
- Scattered photons may also be present in the room and expose the radiographer or radiologist
Recoil electron
happens in Compton scatter
- its the electron that get's ejected as a scattered electron.
- this electron can either ionize other atoms or recombine an ion that needs an electron to be stable
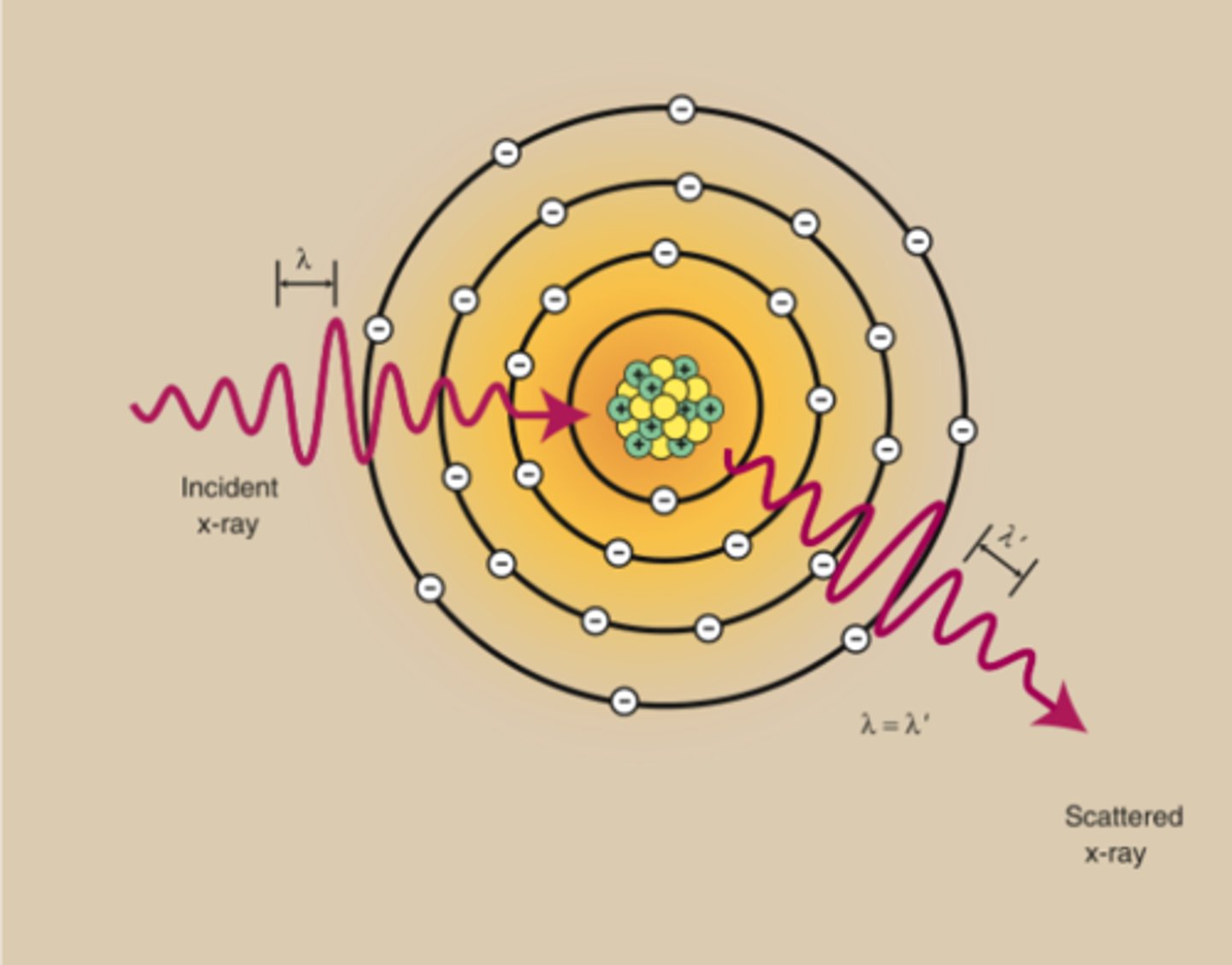
Coherent scatter (interaction)
this interaction;
- occurs in tissues
- also called classical
- Produced by low-energy x-ray photons
- electrons are not removed (this makes it not ionized) but vibrate because of the deposition of energy from the incoming photon (excited)
- As the electrons vibrate, they emit energy equal to that of the original photon. This energy travels in a path slightly different from the path of the original photon. This is what makes it called scatter
- Does not affect image less than 70 kVp
- May have negligible effect on fog greater than 70 kVp