Concept 3.1: Polar covalent bonds in water molecules result in hydrogen bonding
0.0(0)
Card Sorting
1/3
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
4 Terms
1
New cards

Buoyancy
The ability for solids to float on water if their overall density is lower; this includes ice, where water molecules become farther apart
2
New cards
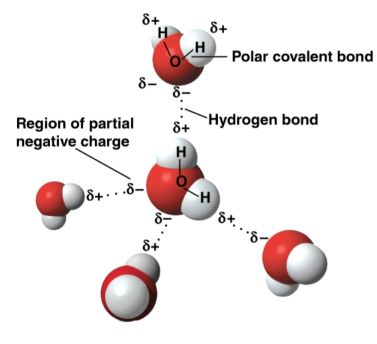
Polar covalent bonds
Bonds created by the hydrogen atoms in water as a result of being attracted towards oxygen
3
New cards
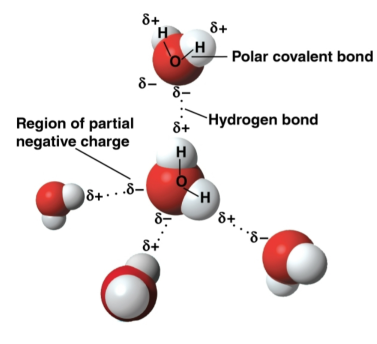
Polar molecule
A molecule where the overall charge is unevenly distributed
Water is an example of this it as it can form hydrogen bonds with other water molecules
4
New cards
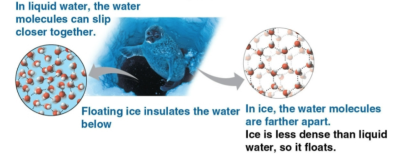
Density
The measure of how much volume a certain amount of mass takes up