Chem: Solids, liquids and gases
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
14 Terms
state 5 changes of state
Melting
freezing
condesation
Evaporation
sublimation
Features of a solid
has a volume and shape
high density
can not be comressed
particles vibrate in the spot
Features of a liquid
No definite volume
cannot be compressed
Can flow
irregular arrangement
particles touch eachother
Features of a gas
Can flow
No definite shape
Can be compressed
Low density
particles move randomly
Define sublimation
This is the change of state directly from a solid to a gas without being a liquid
Define evaporation
The process occuring at the surface of a liquid at any temperature where ther eis a change in state from a liquid to a gas.
Compare and contrast boiling and evaporation
Boiling happens at a specific temperature depending on the substance used while evaporation happens anytime.Evaporation occurs ONLY on the surface while boiling occurs throughout the liquid. However they are both processes that change the substance from a liquid to a solid.
Describe what happens to the particles during Boiling/ evaporation.
1.As we heat the liquid it expands. The particles at the surface that have enough kinetic energy that is given by the thermal energy escape leaving (Evaporation)
As we het the liquid, it expandsthe particles gain eneery from the heat given.and then when they have enough energy to break bonds between them the liquid begins to boil.
Describe the particle theory during melting
As the temperature increases the particles gain more kinetic energy , therefore they vibrate in place more frequent. and so the solid begins to expand and when it reachesit melting point particles have enough energy to break the intermolecular forces between them therefore the solid melts and becomes a liquid.
Describe the kinetic theory of particles suring condensing/ freezing
As te teperature decreases the particles lose kinetic energy therefore vibrations becomes less and less. The liquid stops expanding and the intermolecular forces increases so the particles grow closer together.
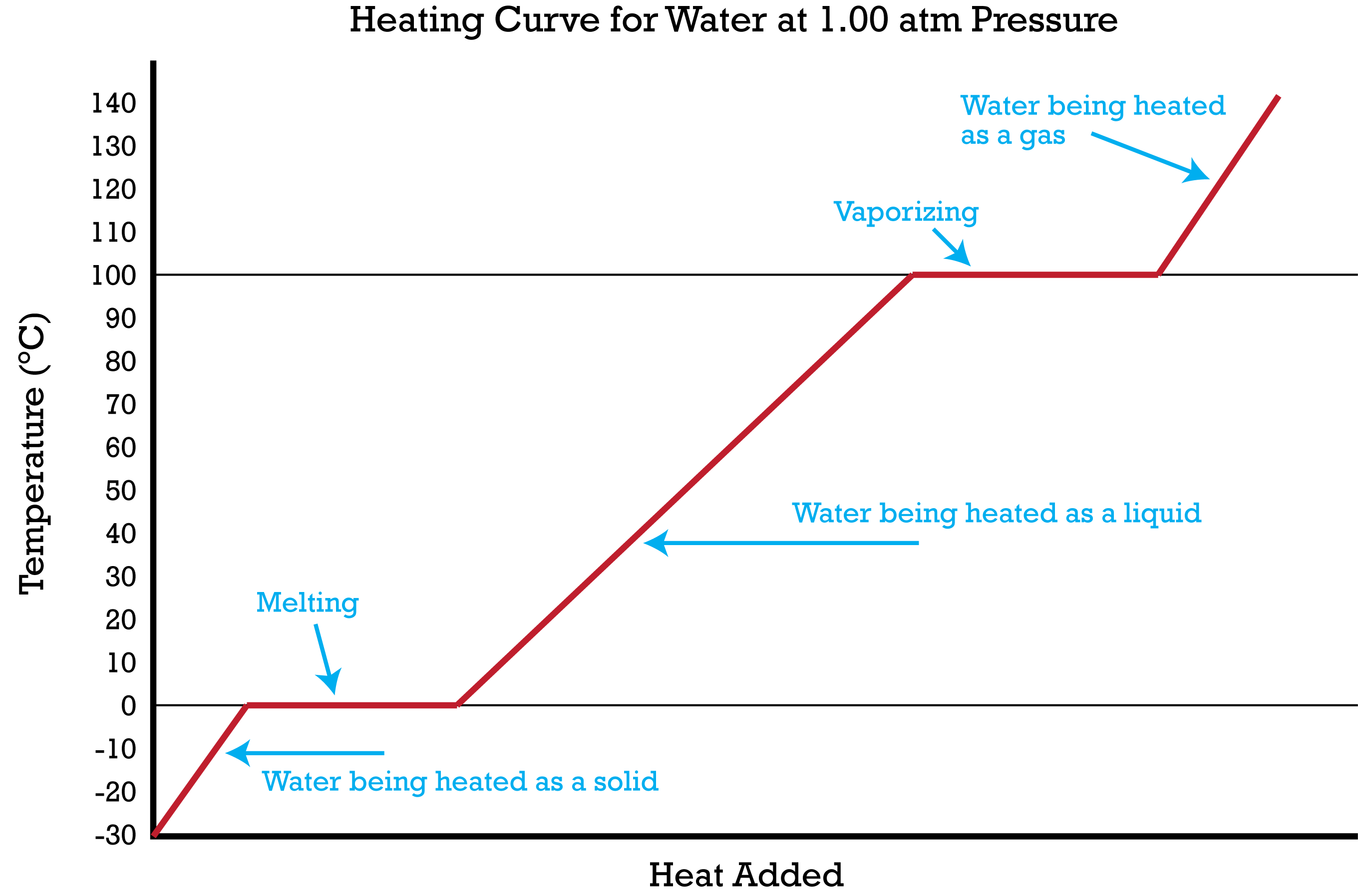
Heating graph
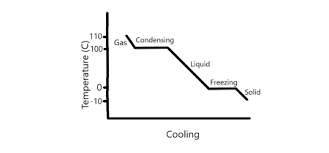
Cooling graph
Explain the effect of increasing temperature on the volume of gas
Increasing the temperature results to the particles gaining kinetic energy therefore the particles will start to randomly vibrate and so the volume increases.
Explain the effect of increasing pressure on the volume of gas
increasing the pressure in a gas the the volume will decrease as we are compressing the gas ,so the particles grow closer to eachother and take up less space.