Unit 1: Reaction Kinetics
1/29
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
30 Terms
Reaction kinetics
Study of the rates of reactions and the factors that affect the rates.
Rate of reaction = speed at which a reaction occurs
Expressed in terms of the change in amount of a reactant (consumption/ decrease) or product (production/ increase) in a certain interval of time.
Mass change
Open system, the NO2 (g) escapes, total mass of system decreases.
Electronic balance and stopwatch.
Rate = Δmass/Δtime
Colour change
Spectrophotometer and stopwatch
Colour intensity increases as more Cu(NO3)2 (aq) is produced.
Rate = Δcolour intensity/Δtime
Pressure change
Closed (sealed) container, a pressure gauge and stopwatch.
To measure pressure change, there must be a change in the total number of moles of gas in the reaction.
Rate = Δpressure/Δtime
Temperature change
Exothermic = temperature increases
Endothermic = temperature decreases
Thermometer and stopwatch
Rate = Δtemperature/Δtime
pH change
pH meter and stopwatch
HNO3 (aq) is used up, acidity decreases and pH of the solution increases
Rate = ΔpH/Δtime
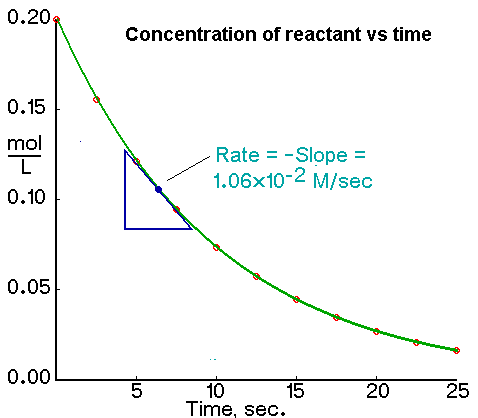
Concentration-Time graph
Rates of reaction do not typically remain constant for the entire duration of a reaction.
Initially, rates are fast because [reactants] are high.
Rates decrease as reaction proceeds since [reactants] decrease.
The exact rate at any particular time can be obtained by determining the slope of a line that is tangent to the concentration-time curve at that point.
Some reactions may show an increase in rate after a slow start
exothermic reactions
changes in surface area
coatings on the reactant surface
autocatalysis
TEMPERATURE affects reaction rates
When temperature is increased, the time required for a reaction decreases.
Rate = Δamount of reactant or product/Δtime
Increasing temperature increases the rate.
In general, for many SLOW reactions, a 10°C increase in temperature doubles the reaction rate.
CONCENTRATION affects reaction rates
Increasing reactant concentration increases rate.
PRESSURE affects reaction rates
Partial pressure of a gas is proportional to the moles of a gas when temperature is constant.
Increasing partial pressure of a gas is equivalent to increasing concentration; therefore, rate increases.
NATURE OF REACTANTS affects reaction rates
Some reactions are naturally faster than others.
Reactions that involve breaking weak bonds or transferring electrons that are weakly held are faster than those in which bonds are strong and electrons are held strongly.
These are fundamental differences in the chemical reactivity of different substances which we have no control.
SURFACE AREA affects reaction rates
Heterogeneous reaction = reactants are in different phases (i.e., solid and liquid or solution)
Homogeneous reaction = reactants in the same phase (e.g., two gases).
Increasing exposed area (surface area) increases the rate.
Crushing, powdering, grinding, etc.
Only affects the rate of heterogeneous reactions.
CATALYSTS AND INHIBITORS affect the reaction rates
Catalyst = chemical which reduces reaction rate but is regenerated in its original form at the end of a reaction.
Inhibitor = chemical substance that reduces reaction rate by combining with a catalyst or one of the reactants in such a way that it prevents the reaction from occurring.
PHASE OF REACTANTS affects the reaction rates
Oppositely charged aqueous ionic reactants lead to very fast reaction rates.
Few bonds or weak bonds between reactants have faster rates than many and strong ones.
Homogeneous reactants have faster rates than heterogeneous phases.
Undergoing 2-particle collisions is faster than those involving 3 or more particles.
Order of reaction rate (fastest → slowest): Aqueous ions > Gases or Liquids > Solids
Particles in a solid do not have free movement.
Particles in a solution are close and have free movement.
Collision Theory (Kinetic Molecular Theory)
Reactions depend on collisions between reactant molecules; however, not all collisions lead to a reaction.
Successful or effective collisions lead to the formation of products, while those that don’t are ineffective or unsuccessful.
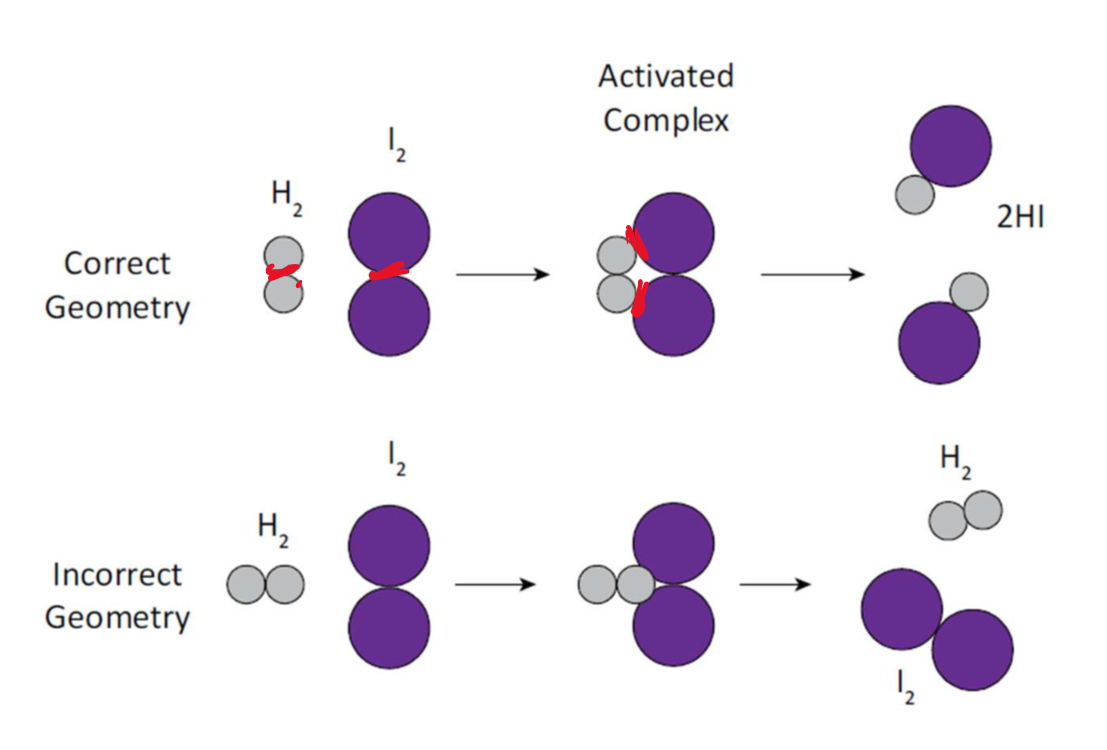
Orientation or geometry
Colliding reactant molecules must be oriented in a favourable position to allow bonds to break and atoms to rearrange.
Activation energy
For a reaction to occur, molecules need to collide with sufficient energy to break bonds so that atoms can rearrange and form new bonds.
Activation energy = minimum amount of energy needed for a reaction to occur.
Collision Theory - Increasing Concentration
Increasing the concentration of reactants (or partial pressure if gases) increases the frequency of possible collisions and hence increases the rate.
The percentage or fraction of collisions that are effective remains the same.
Collision Theory - Increasing Temperature
Increasing temperature increases the average kinetic energy of the molecules (i.e., increases the speed at which the molecules are moving).
Two effects:
molecules collide more often
molecules collide with more energy
Increasing temperature increases the percentage or fraction of effective collisions.
Rate increase that accompanies an increase in temperature is primarily due to the increased energy of collisions.
Potential energy
Stored energy
Related to the energy of the electrons in the chemical bonds, as well as the number and types of atoms in the molecule.
Potential energy increases when bonds are broken and decreases when new bonds are formed.
To break a bond, energy must be put into the reaction
To form a bond, energy is released
Kinetic energy
Energy of motion
Energy exists as a result of a movement of molecules within a system.
Kinetic energy can be related to the temperature of the system.
Enthalpy (H)
Heat of reaction
TOTAL kinetic and potential energy that exists in a system at constant pressure.
ΔH = Hproducts - Hreactants
During a chemical reaction, the bonds of the reactant molecules are broken, the atoms are rearranged, and new bonds are formed.
Exothermic reaction: Hproducts < Hreactants
ΔH < 0
Heat is released into the surroundings → Warmer temperature
Endothermic reaction: Hproducts > Hreactants
ΔH > 0
Heat is absorbed into the system → Cooler temperature
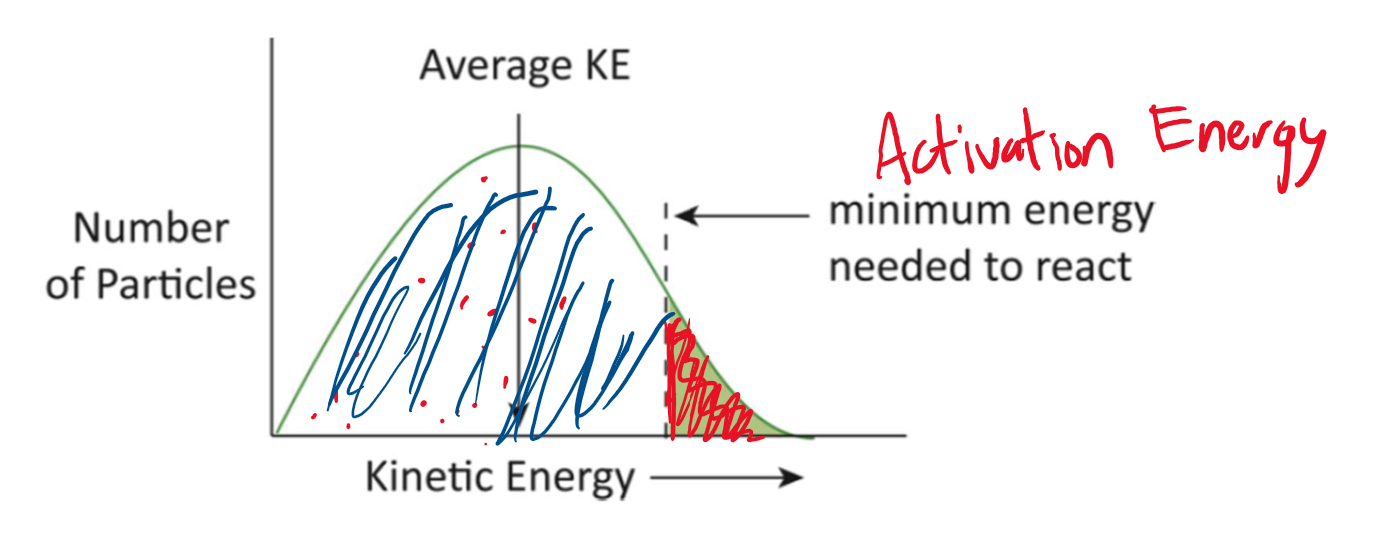
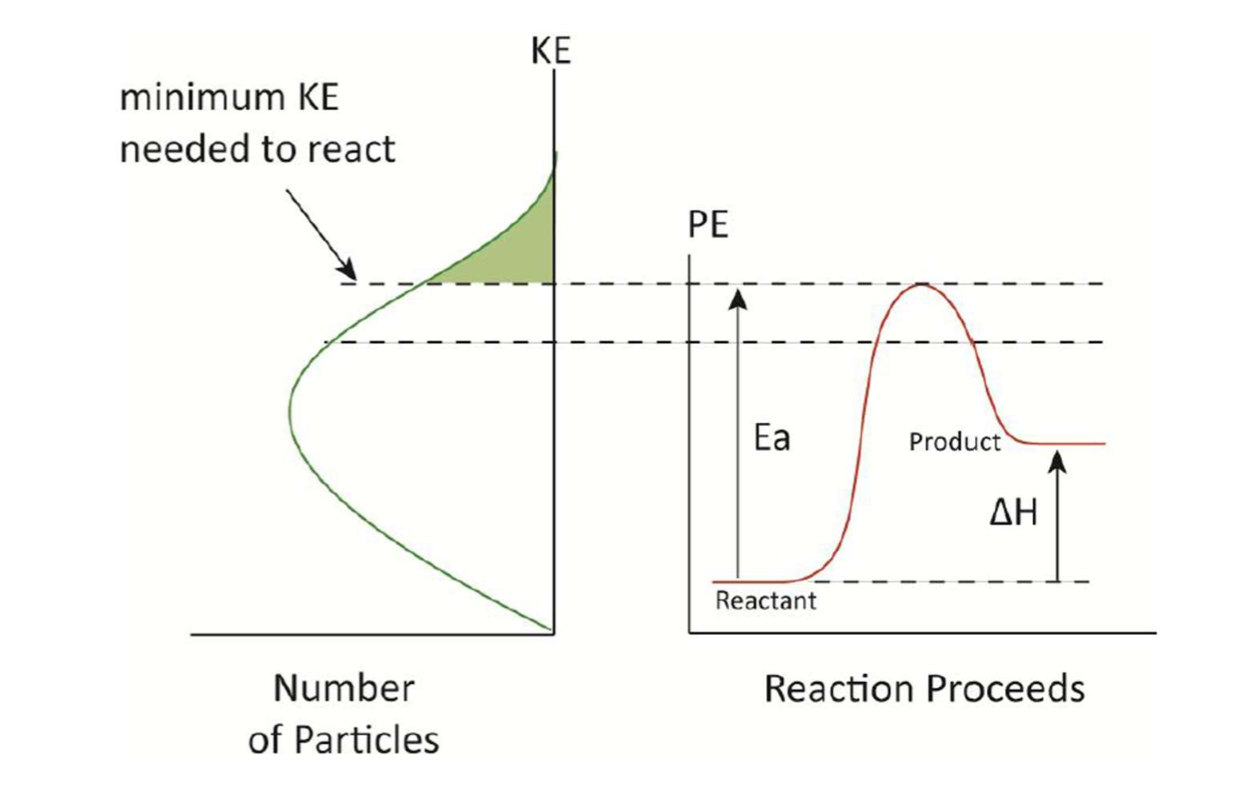
Kinetic energy distribution (Maxwell-Boltzmann distribution)
Room temperature and pressure: molecules undergo about 1010 collisions/second.
→ The lack of reactivity is NOT due to the lack of collisions.Temperature increases → Molecules have more energy → Reaction rate increases.
Some molecules have high KE while others have low KE.
Increasing temperature increases the average energy of the system.
Only the molecules with KE ≥ minimum energy will react.
The increased reaction rate due to an increase in temperature is PRIMARILY DUE to the increased number of molecules with sufficient energy to react.
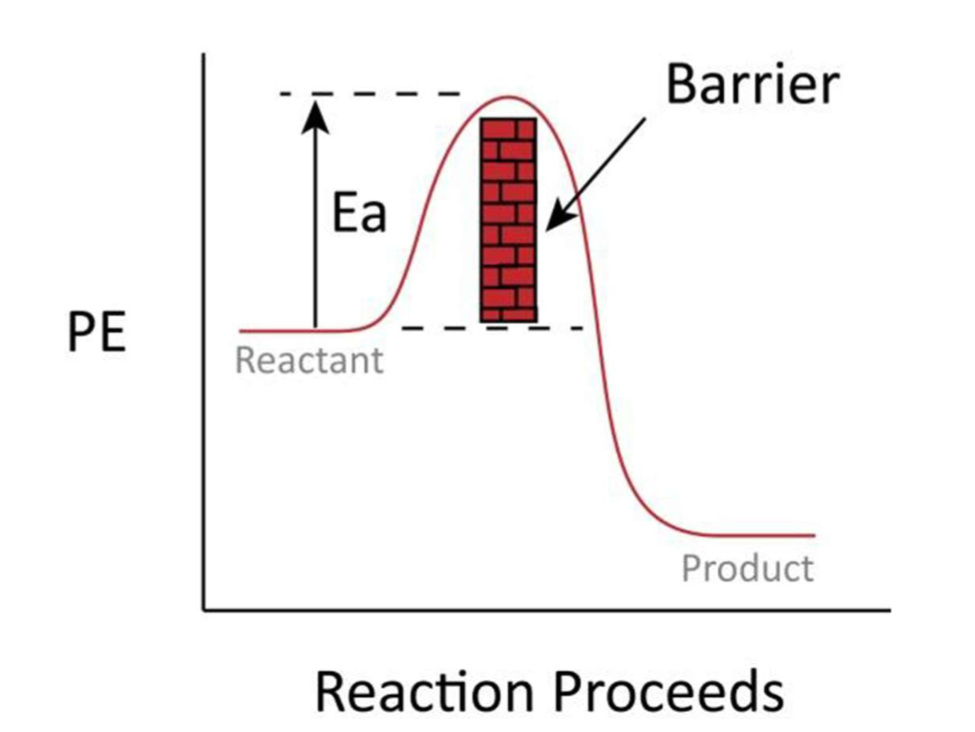
Activation energies
Activation energy (Ea) = the minimum amount of energy required for the reactants to form the activated complex.
Reactant molecules approach each other → Slow down → KE is converted into PE.
e-repulsion → e- gain PE by absorbing KE → slow down the reaction → reactant molecules gain enough energy (≥ Ea) → activated complex is formed → e-repulsion → product molecules move away from each other → PE is converted into KE.
Activated complex
High energy, unstable arrangement of atoms which occurs when reactants are in the process of rearranging to form new products.
Total energy = PE + KE
Molecules do not have ideal geometry → Reaction takes place with an increased activation energy.
Ea = Activated Energy - Hreactant
ΔH = Hproducts - Hreactants
Reversible reaction
Reactant ⇆ Product
Ea(fwd): activation energy for the forward reaction (reactant to activated complex).
Ea(rev): activation energy for the reverse reaction (products to activated complex).
Ea(fwd) = Ea(rev) + ΔH (ΔH > 0)
Ea(rev) = Ea(fwd) + ΔH (ΔH < 0)
The higher the activation energy, the slower the reaction rate, and vice versa.
High Ea = Low reaction rate
Low Ea = High reaction rate
Reaction mechanism
Reactions often occur as a result of several elementary steps.
Elementary process = individual step in a reaction mechanism.
Reaction mechanism = sequence of steps that make up an overall reaction.
Each step has its own peak.
Overall activation energy: the difference between the reactants and the highest peak.
Activation energy for each step is the PE difference between the activated complex and the reactants involved in that step.
Rate-determining step = the slowest step in a mechanism (highest Ea).
Effects of catalysts on Ea
Catalysts do NOT change the ΔH.
Catalysts lower the activation energy → More molecules have enough energy to react → Reaction rate increases.
Catalysts decrease both the Ea(fwd) and Ea(rev).
Catalysts work by providing an alternative reaction mechanism with a lower Ea.
Reaction intermediate vs. Catalyst
Reaction intermediate: product → reactant
Catalyst: reactant → product
Both intermediate species and catalyst cancel out when the individual steps are added to get the overall reaction.