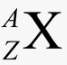Unit 4 - Atomic Models
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
19 Terms
John Dalton
father of chemical atomic theory.
Proposed that elements made of particles are called atoms
Atoms of the same element have same properties at
Atoms of different elements have different properties
Law of Definite Proportions
Atoms can combine in whole number ratio
Law of Multiple Proportions
Atoms of the same element can unite in more than one ratio with another elementt to form more than one compound1
Atom
The smallest particle of an element that retains the properties of the element
F
(T/F) The atom is indestructible and indivisible.
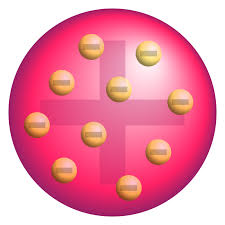
Plum Pudding Model; JJ Thomson; positive sphere of matter with negative electrons in it
Name; Who; What
Atomic Number
Number of protons
Atomic mass
The mass of one atom of an element relative to one atom of another element.
Mass number
Sum of protons and neutrons in an atom
Isotopes
Atoms of the same element but with a different mass number because of a different amount of neutrons
Atomic mass
___ of an element is the weighted average mass of the isotopes of that element.
Percent abundance
how much of that isotope there is out of that element
Average atomic mass = (% Abundance 1)(mass 1)+(% Abundance 2)(mass 2)
Average atomic mass formula
Leucippus and Democritus
First to propose that matter made of particles that could not be cut up any further. He called them “atomos”.
Aristotle
Proposed all matter consisted of four elements, earth, water, air, fire.
JJ Thomson
Used cathode ray to study atoms. He showed that there were smaller particles in atoms. Those charged particles were electrons.
Ernest Rutherford
Show particles in a thin sheet of gold foil and observed the pattern of scatter of the particles. He proposed that there was a very small dense nucleus with the electrons around the atom.
Isotope name
Element-Number
Isotope symbol