CHEM 1211 Section 4.1 (Chemical Bonds and Greenhouse Gases
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
18 Terms
valence
the capacity of atoms of a particular element to form chemical bonds
valency
Some elements exhibit more than one _______ because they form different numbers of chemical bonds.
Electrostatic Potential Energy (Eel)
the strength of electrostatic attractions between ions of opposite charges
Coulombic Attraction
another term for electrostatic potential energy
directly
The value of Eel between a pair of ions is________ proportional to the product of their charges (Q1 and Q2)
inversely
The value of Eel between a pair of ions is _________ proportional to the distance (d) between their nuclei
Coulomb
the SI unit of electrical charge
repel
Two ions of the same charge _____ each other.
positive
When two positive or two negative ions repel each other, a ________ Eel is created.
negative
When a negative ion and a positive ion attract each other, a ________ Eel is created.
greater
The _______ the attraction between ions, the more negative the Eel value is.
Avogadro Constant
6.022×10²³
Metals
shiny, solid conductors that are malleable and ductile
Nonmetals
brittle solids, liquids and gases that are nonconductors
Metalloids
Shiny but brittle
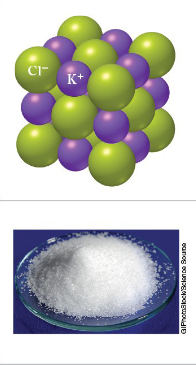
Ionic Bond
A bond that includes metals with nonmetals. Electrons are transferred

Covalent Bond
A bond that includes nonmetals with nonmetals (and/or metalloids). Electrons are shared

Metallic Bonds
A bond that includes only metals. Electrons are delocalized