DIG Exam 3 Microbiota Audia
1/50
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
51 Terms
Microbes possess biochemical pathways to metabolize substrates into a form that is _____ by the human host
useable
Microbes produce compounds that we are ____ to produce for ourselves
unable
Microbes set up a niche that is fiercely competitive, limited for space, and nutrients protecting us from colonization by ______ microbes
pathogenic
Hygiene
Diet
Stress
Antibiotics
Influence our microbiome
Lowest microbial load
Helicobacter pylori in some individuals
Lactobacillus species
Stomach and duodenum
Acid tolerant organisms
Bile salts limit growth
Streptococcus, lactobacillus, Enterobacteriaceae
Alkaline 8-10
Jejunum
Facultative anaerobes
pH 5-7
Reduced bile salts
Mixed facultative and anerobic organisms
Transition to anaerobic environment
Ileum
Densest microbial community
90% flora Bacteroidetes (gram - rods) and firmicutes (gram + rods)
Key species Bacteroides fragilis, Bacteroides thetaiotaomicron
Colon
strictly anaerobic environment
Dest complex carbs
Majo contributor to colonic fermentation and SCFA production
B theraiotaomicorn
B fragilis
Microbiome fermentation of dietary fiber to ____
SCFAs
SCFA, primary energy source of colonocytes; maintains barrier integrity
Butyrate
SCFA, travels to liver; GNG substrate
Propionate
SCFA circulates systemically, influences lipid metabolism
Acetate
Vitamin K, folate, biotin
Vitamin synthesis by microbiome
Deconjugation and transformation, regulates lipid absorption and signals via FXR/TGR5 pathways
Bile acid metabolism by microbiome
Gut flora can contribute up to ____-___% of host daily calories through fermentation of otherwise indigestible carbs
10-15
SCFAs interact with host GPCRs to regulate appetite, insulin sensitivity, and inflammation
Metabolic signaling with microbiome contribute to
Microbiomes promote balanced Th1/Th2/Th17 responses
Commensals
SCFA especially butyrate enhances _____ differentiation in the colon. Critical for dampening inflammation and maintaining mucosal tolerance.
Treg
Stimulates dendritic cells to promote Treg development and IL-10 production
Protects against experimental colitis in animal models
B fragilis polysaccharide A (PSA)
Shaped by microbial interactions, reinforces mucosal barrier defense
Secretory IgA
B fragilis colonization restores IL-10 production in a ____ dependent manner
PSA
Commensals provide molecular signals like PSA to actively _____ mucosal tolerance
induce
IL-10 induction occurs almost exclusively in _____ Tregs
Foxp3
A 67-year-old woman presents to her physician complaining of persistent diarrhea with weight loss, bloating, and excess flatulence (passing gas). She also mentioned her stools looked ‘greasy’, which the physician knew as a sign that ingested fat was not being absorbed. A blood test revealed the woman’s B12 level was abnormally low. Cultures of fecal specimens failed to detect any typical bacterial pathogens that could cause diarrhea. What is going on?
Decreased vitamin B12 level and excess fat in the stool suggested the woman had a malabsorption disorder
Possible cause small intestine bacterial overgrowth disease
Characterized by aspirating fluid from the jejunum and finding abnormally high numbers of facultative and anaerobic bacteria such as Streptococcus, E. coli, Lactobacillus and Bacteroides
Fermentation by these organisms produced the bloating and flatulence Antibiotic treatment corrected the syndrome
Decreased stomach acidity
Reduced peristalsis
Mucosal damage or atrophy
Causes of SIBO
Bacteroides can deconjugate bile acids; less bile leads to malabsorption of fatty acids because micelles do not form
Deconjugated bile acids also inhibit carbohydrate transporters, so sugars accumulate and bacteria ferment them
Fermentation by these organisms produced the bloating and flatulence
The organic acids make lumen pH acidic which helps induce osmotic diarrhea
Deconjugated bile acids also damage enterocytes, leading to water loss
Series of events for SIBO
Rifaximin (RNA polymerase inhibitor)
Augmentin (Ampicillin + Clavulanic acid cell wall synthesis inhibitor)
SIBO treatment
A 24-year-old man presents with several months of abdominal pain, diarrhea, and weight loss. He describes his stools as loose and occasionally containing blood. On physical examination, there is tenderness in the right lower quadrant. Laboratory studies show an elevated C-reactive protein and anemia. Colonoscopy reveals patchy areas of mucosal ulceration separated by normal-appearing mucosa. Biopsies demonstrate chronic inflammation extending through the bowel wall. Stool cultures are negative for bacterial pathogens.
Inflammatory Bowel Disease (Crohn’s, UC)
Reduced microbial diversity
IBD patients show decreased overall gut flora diversity
Reduced protective commensals (e.g., Faecalibacterium prausnitzii)
Increased pro-inflammatory taxa (e.g., Enterobacteriaceae)
Mechanistic relevance
Dysbiosis promotes barrier disruption and aberrant immune activation (Th17 > Treg imbalance)
Dysbiosis contributes to chronic, relapsing inflammation in Crohn’s disease and ulcerative colitis (not caused by an infection)
____ patients show decreased overall gut flora diversity
IBD
Increased pro-inflammatory taxa
Enterobacteriaceae
Reduced protective commensals
Faecalibacterium prausnitzii
Dysbiosis promotes barrier disruption and aberrant immune activation (Th17 > Treg imbalance)
contributes to chronic, relapsing inflammation in Crohn’s disease and ulcerative colitis
Reduced microbial diversity and loss of protective commensals
IBD
Underrepresentation of firmicutes in ____
IBD
Definition: Imbalance in microbial communities that disrupts normal host -microbe symbiosis
Dysbiosis
Altered microbiota can increase energy harvest from diet
Phenotype shown to be transmissible in germ-free mouse models (microbiota transplant)
Obesity and the microbiome
A 42-year-old female presents to the Emergency Department (ED) complaining of passing profuse green-colored loose stools she describes as foul smelling and ‘greasy and bloody’ in appearance. She also complains of frequent cramping and nausea. Patient’s temperature is 104 oF / 40 oC. She reports being treated over the last 2-3 weeks with several different ‘kinds’ of antibiotics as part of a recent surgery but doesn’t recall the specific antibiotics. The patient is admitted and a colonoscopy ordered.
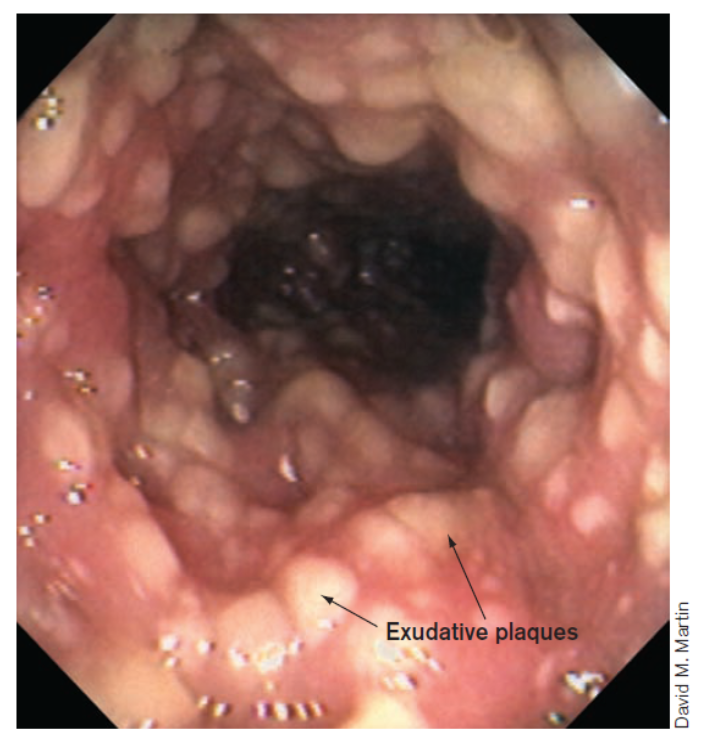
C diff colitis
Antibiotic treatment, especially following surgery, kills off normal intestinal microflora, leaving C. diff to grow unabated
C. diff → toxin → damages and kills host epithelial cells → exudative plaques form on the intestinal wall
The small plaques eventually coalesce to form a large pseudomembrane that can slough off into the intestinal contents
C diff Gram positive spore forming anaerobe
1 to 6 days post antibiotic treatment
Watery Diarrhea
Abdominal cramping
Fever
Leukocytosis
Diarrheas beginning within 3 days after hospitalization
C diff symptoms
Disease precipitated by certain antibiotics to which C. dif is resistant
Clindamycin
Ampicillin/Cephalothin
Fluoroquinolones
C diff is normal microbiota in colon of some people
Causes cytokine release
Chemoattractant for PMNs
Inflammation – Diarrhea (rarely bloody)
C diff enterotoxin TcdA
Cell necrosis
Affects tight junctions
C diff necrotizing toxin TcdB
ELISA to Toxin A, or PCR of stool looking for tox genes
diagnosis for C diff
Remove other antibiotics, then...
Oral Vancomycin or Metronidazole
Oral Rehydration Therapy
Metronidazole- generates ROS, damages DNA (for anaerobic)
Vancomycin inhibits Cell wall synthesis (good for GI localized because it is not absorbed well by the gut)
treatment for c diff
Indication: Recurrent C. difficile infection (after antibiotics fail)
Delivery: colonoscopy, NG tube, capsules (emerging)
Investigational uses: IBD, metabolic syndrome, immune modulation
FMT
Fiber-rich diets support SCFA-producing taxa → promote colonic health and immune tolerance
microbiome modulation
Low-fiber/high-fat diets linked to reduced diversity and pro-inflammatory communities
microbiome modulation
Diet can override microbial composition (e.g., germ-free mice colonized with “obese” microbiota but given a fiber-rich diet resist weight gain)
Microbiome modulation
Examples: Lactobacillus, Bifidobacterium
Shown to reduce risk of antibiotic-associated diarrhea in some Randomized Control Trials (RCTs)
Variable or minimal benefit in IBD or obesity
Efficacy is strain- and condition-specific, not “one size fits all”
Transient colonizers
Most probiotic strains do not permanently integrate into the gut microbiome
They pass through the GI tract and decline once supplementation stops
Competition with resident microbiota
Native communities have established niches; probiotics rarely displace them long-term
Detectable only during active ingestion; levels fall rapidly after discontinuation
Daily intake is needed to maintain probiotic effect — benefits are linked to presence, not permanent colonization
Probiotics