period 3 oxides - trends and reactions w/ water
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
24 Terms
describe and explain the overall trends in the mpts of the period 3 oxides from Na2O → Al2O3:
increase, decrease (respectively)
overall: high mpts as form giant ionic lattices - strong electrostatic forces of attraction require a large amount of E to overcome
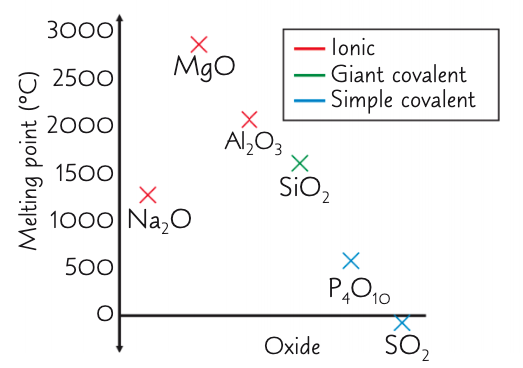
how and why does the mpt change from Na2O to MgO?
increase
Mg is smaller and has a bigger charge and so a greater charge density
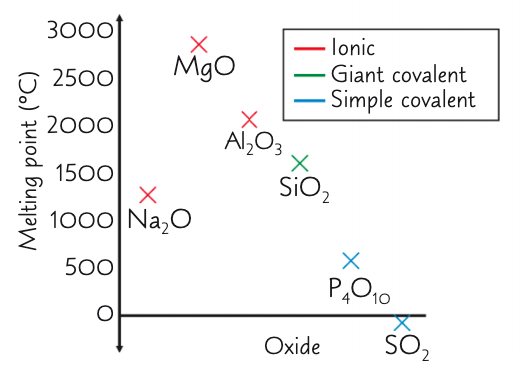
how and why does the mpt change from MgO to Al2O3?
decrease
smaller electronegativity diff between Al and O than Mg and O
so oxygen ions in Al2O3 don’t attract e- in the metal-oxygen bond as strongly as in MgO
so Al2O3 bonds are partially covalent
how and why does the mpt change when you reach SiO2?
decrease - but still higher compared to other non metal oxides
as has a giant covalent structure with strong covalent bonds which require a large amount of energy to break
describe and explain the overall trend in the mpts of the period 3 oxides from P4O10 → SO2:
decrease, decrease (respectively)
low mpts as are simple molecular structures - weak IMF: DPDP and VDWs which require little energy to overcome
how do Na2O and MgO react with water? refer to the ions and state the ionic eqn:
dissolve in water
O2- ions react with water to form OH-, forming alkaline solutions
O2- + H2O → 2OH-
give the eqn for Na2O reacting with water:
Na2O (s) + H2O (l) → 2NaOH (aq)
give the eqn for MgO reacting with water and state the additional observation:
MgO (s) + H2O (l) → Mg(OH)2 (aq) - bright white flame
are Na2O and MgO acidic, amphoteric or basic oxides? why?
basic - react with acids (to form bases)
which 2 group 3 oxides are insoluble in water and why?
Al2O3 and SiO2
dessicants - absorb water into structure
is SiO2 basic, acidic, or amphoteric? what does this mean?
acidic - reacts with bases (to form acids)
are sulfur oxides acidic, basic or amphoteric? what does this mean?
acidic - react with bases (to form acids)
give the eqn for the reaction of phosphorous oxide with water:
P4O10 (s) + 6H2O (l) → 4H3PO4 (aq)
give the eqn for the reaction of sulphur dioxide with water and name the product:
SO2 (g) + H2O (l) → H2SO3 (aq) - sulphurous acid
give the eqn for the reaction of sulfur trioxide with water and name the product:
SO3 (g) + H2O (l) → H2SO4 (aq) - sulphuric acid
is silicon dioxide soluble in water? is it acidic, basic or amphoteric?
insoluble
acidic - reacts with bases (to form acids)
is aluminium oxide water soluble? is it acidic, basic or amphoteric?
insoluble
amphoteric - reacts with both acids and bases
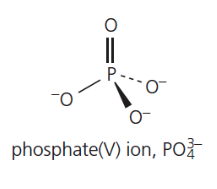
give the formula and structure of the phosphate (V) ion:
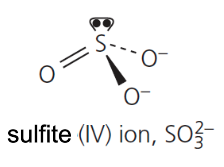
give the formula and structure of the sulfite (IV) ion:
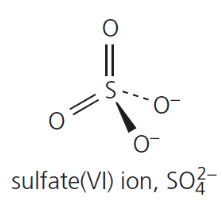
give the formula and structure of the sulfate (VI) ion:
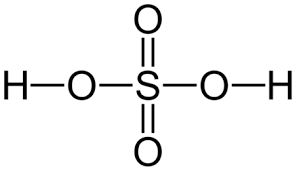
give the formula and structure of sulfuric acid:
H2SO4
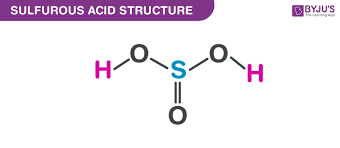
give the formula and structure of sulfuruous acid:
H2SO3
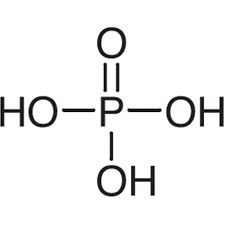
give the formula and structure of phosphoric acid:
H3PO4
give the formula of a hydroxide of an element in period 3 used in medicine:
Mg(OH)2