Chemistry Quarter 1 test
1/39
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
40 Terms
Homogeneous
The composition is the same throughout
Heterogeneous
The composition is not the same throughout
Atom
The smallest particle of an element that still has the element’s properties
Molecule
The smallest particle of a covalent compounds that still has the properties of the compound
Element
The simplest form of matter that has a unique set of properties
Compound
A substance that contains two or more elements chemically combined in a fixed proportion
Mixture
A physical blend of two or more compounds, each of which retains their own identity and properties
Substance
Matter that has a uniform and definite composition
Mass
The amount of matter present in an object
Volume
The space matter combines
Temperature
A measure of the average kinetic energy
Density
The amount of mass per unit volume
Intensive property
A property that does not depend on the amount of matter present
Extensive property
A property that does depend on the amount of matter present
Identifying property
A specific characteristic of a substance, whether physical or chemical, that allowed one to distinguish it from other substances
Accuracy
How close a measurement is to an accepted value
Precision
How close to a set of measurements are to each other
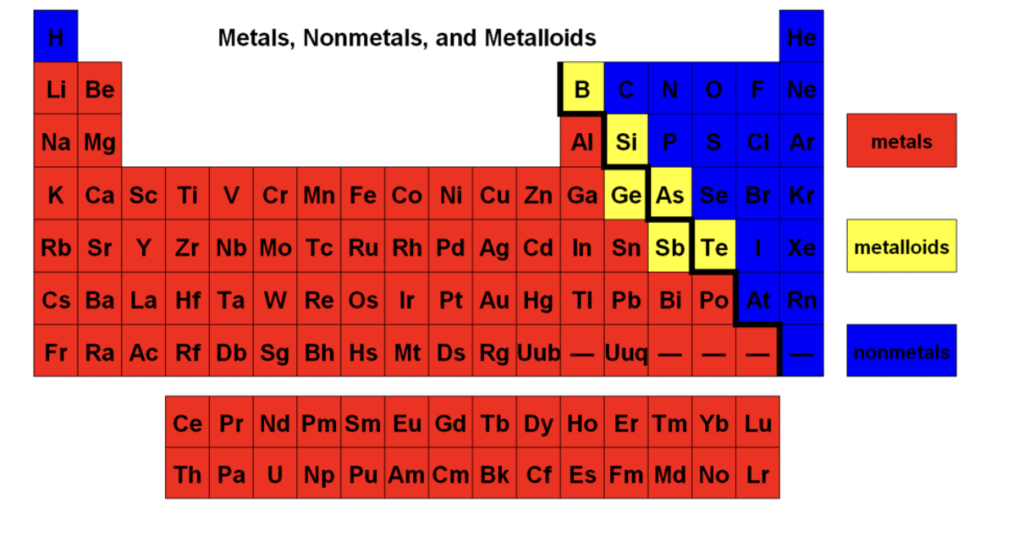
Metal
Material that is conductive, malleable, ductile, and has luster
Metalloids
Materials that have properties of both metals and nonmetals. Many are semiconductors
Nonmetals
Materials that tend to be brittle, nonconductive, and dull
Group
One of eight columns on the periodic table. First 2 and last 6
Family
One of eight columns on the periodic table. First 2 and last 6
Period
A row on the periodic table
Series
A row on the periodic table
Chemical reaction
Chemical combining, breaking apart, or rearranging. A new substance is formed
Chemical properties
Properties of matter that can only be determined by carrying out a chemical reaction
Physical properties
Properties of matter that can be determined without carrying out a chemical reaction
Reactants
The chemicals that are going to react and are on the left side of a chemical reaction
Products
The chemicals that are produced in a chemical reaction and are on the right side of a chemical reactions
Malleable
Materials that can be pound into a very thin sheet
Conductivity
How easily electrons or heat flow through a substance
Luster
Shininess
Ductile
Ability to be drawn into a wire
Solid
Matter that has a definite volume or shape
Liquid
Matter that has a definite volume and an indefinite shape
Gas
Matter that has an indefinite volume and an indefinite shape
Metals, nonmetals, and metalloids on periodic table
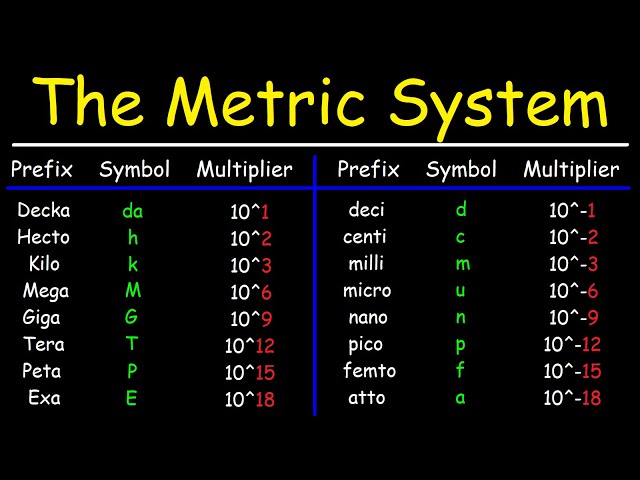
Metric conversions
Mole
6.02×10²³
Why is a mole a mole?
It is the exact amount of atoms in 12 grams of C-12. C-12 is the most abundant of the two stable isotopes of carbon