Chapter 21 - Nuclear Chemistry
1/63
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
64 Terms
Radioactivity
Emission of subatomic particles or high-energy electromagnetic radiation by the nuclei of certain atoms
Phosphorescence
Long-lived emission of light by atoms or molecules that sometimes occurs after they absorb light
Uranic rays
Isotope
Atoms of the same element with different numbers of neutrons
Mass number formula
Mass number = number of protons + neutrons
Isotopic notation (nuclide)
AZX (where A = mass number, Z = atomic number, and X = chemical symbol)
Proton symbol
Regular symbol: p+; nuclear symbol: 11H, 11p
Neutron symbol
Regular: n0; nuclear: 10n
Electron symbol
Regular: e-; nuclear: 0-1e
Alpha symbol
Regular: α; nuclear: 42α, 42He
Beta symbol
Regular: β, β-; nuclear: 0-1β, 0-1e
Positron symbol
Regular: β, β+; nuclear: 0+1β, 0+1e
Alpha rays
Have a charge of +2 and mass of 4 amu (what we now know to be helium nucleus)
Beta rays
Have a charge of -1 c.u. and negligible mass (electron-like)
Gamma rays (γ)
Form of light energy (not a particle like α and β)
Positron
Charge of +1 and negligible mass (antiparticle of electron), some unstable nuclei emit positrons
Electron capture
When an inner orbital/low energy electron is pulled into the nucleus (also action of some unstable nuclei), no particle emission, but atom changes (same result as positron emission)
When a proton combines with the electron to make a neutron:
Mass number stays the same, atomic number decreases by 1
Nuclear equations
Atomic numbers and mass numbers are conserved
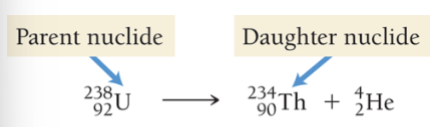
Alpha decay
Occurs when an unstable nucleus emits a particle composed of two protons and two neutrons, most ionizing but least penetrating
Loss of an alpha particle means:
Atomic number decreases by 2 and mass number decreases by 4
Beta decay
A neutron becomes a proton when an unstable nucleus emits an electron, about 10 times more penetrating than alpha decay, but only about half the ionizing ability
Loss of a beta particle means:
Atomic number increases by 1, mass number remains the same
Gamma emission
No loss of particles from the nucleus, no change in composition of the nucleus (same atomic # and mass #), least ionizing but most penetrating, generally occurs after the nucleus undergoes some type of decay and the remaining particles rearrange
Ionizing power
The ability of radiation to ionize other molecules and atoms
Penetrating power
The ability to penetrate through matter
Positron emission
A proton becomes a neutron when a positron is emitted from its nucleus
When an atom loses a positron:
Mass number remains the same and atomic number decreases by 1
Strong force
A very strong attractive force holding the particles in the nucleus together (lol???), acts over only very short distances
N/Z ratio
The ratio of neutrons:protons
If N/Z ratio is too high:
Neutrons are converted to protons through beta decay
If N/Z ratio is too low:
Protons are converted to neutrons via positron emission or electron capture (or alpha decay, though not as efficiently)
Valley of stability
For Z = 1-20, stable N/Z ~ 1; for Z = 20-40, stable N/Z approaches 1.25; for Z = 40-80, stable N/Z approaches 1.50; for Z > 83, there are no stable nuclei
Magic numbers
Decay series
Atoms with Z > 83 are radioactive, one radioactive nuclide changes into another radioactive nuclide, all radioactive nuclides are produced one after the other until a stable nuclide is reached
Ways of detecting radioactivity
Thermoluminescent dosimeters, Geiger-Muller counter, scintillation counter
Constant half-life
Length of time for a radionuclide required to lose half its radioactivity
Kinetics of radioactive decay
Rate of change in amount of radioactivity is constant and different for each radioactive isotope, constant half-life follows first-order kinetics, not affected by temperature
Rate
kN (N = # of radioactive nuclei)
Half-life formula
t1/2 = 0.630/k
The shorter the half life:
the more nuclei decay every second; therefore we say the sample is hotter
Radioactive decay formula
ln(Nt/N0) = -kt (Nt = # of radioactive nuclei at time, t; N0 = initial # of radioactive nuclei) (can also be written as Nt = N0e-kt)
C-14 half-life
5730 years
Radiocarbon dating
While still living, 14C/12C is constant because the organism replenishes its supply of carbon. Once the organism dies, the 14C/12C ratio decreases. The half-life of 14C is 5715 years.
Radiometric dating (slides 55 and 56)
Nuclear fission
A large nucleus splits into two smaller nuclei via reaction with neutron
Fusion
Small/light nuclei can be accelerated to smash together to make a larger/heavier nucleus, energy source of stars, basis for hydrogen bombs, requires high energy input to initiate the reaction (need to overcome repulsion of positive nuclei)
Does fission or fusion release more energy per gram?
Fusion releases 10 times more energy per gram as fission with less problematic products
fissionable material (slide 59)
Fission chain reaction
Occurs when a reactant in the process is also a product, in the fission process it is the neutrons
Critical mass
The minimum amount of fissionable isotope needed to sustain the chain reaction
Nuclear power plant
Uses about 50 kg of fuel to generate enough electricity for 1 million people, no air pollution
Coal-burning power plant
Uses about 2 million kg of fuel to generate enough electricity for 1 million people, produces CO2 (greenhouse gas), NO2, and Sox (acid rain)
Problems with nuclear power
Core meltdown (water loss from core; heat melts core), waste disposal (highly radioactive waste)
Energy from fission equation
E = mc2, each mole of U-235 that fissions produces about 1.7 × 103 J of energy, a very exothermic chemical reaction produces 106 J per mole
Nuclear transmutation
When atoms of one element are transformed into atoms of a different element by nuclear reaction, usually involve accelerated high-energy particles smashing into target nuclei
Artificial transmutation
Bombardment of one nucleus with another/neutrons causing new atoms to be made (can be done in a particle accelerator)
Three types of radiation effects
Acute radiation damage, increased cancer risk, and genetic effects
Acute effects of radiation
High levels of radiation over a short period of time kill a large number of cells, causes a weakened immune system and lower ability to absorb nutrients from food
Exposure
Number of decay events to which a person is exposed
Dose
Amount of energy actually absorbed by body tissue
Units of radiation exposure
1) Curie (Ci) = 3.7 × 1010 events per second no matter the kind of radiation
2) Gray (Gy) measures amount of energy absorbed by body tissue, 1 Gy = 1 J/kg body tissue
3) Rad also measures amount of energy absorbed by body tissue, 1 rad = 0.01 Gy
Relative biological effectiveness (RBE)
A correction factor used to account for a number of factors that affect the result of the exposure, result is the dose in rems (roentgen equivalent man), dose in rads x RBE = dose in rems
Roentgen
Amount of radiation that produces 2.58 × 10-4 C of charge per kg of air