ANTI CANCER
1/265
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
266 Terms
Characteristics of cancer
• Uncontrolled proliferation (autonomous)
• Dedifferentiation and loss of function
• Tissue invasiveness-metastasis
FOUR Differences between cancer and infection
Infections Involve a Biologically Foreign Microbe
Infections are caused by external pathogens (bacteria, viruses, fungi, etc.) that are not part of the host.
Pathogen Metabolism Differs from Host Cells
Microbes have distinct metabolic pathways, making them easier to target selectively.
Selective Action of Chemotherapeutic Agents
Antimicrobials can often kill/inhibit microbes without damaging host cells due to this metabolic difference.
In contrast, anticancer drugs may also affect normal dividing cells (e.g. hair follicles, GI lining).
Host Immune System Aids in Defense
In infections, the body mounts a strong immune response using:
Antibodies
Phagocytosis
Cancer often evades or suppresses the immune response, making defense more difficult.
Factors influencing tumor genesis
Gene Mutations
Core driver of cancer development.
Includes activation of oncogenes and inactivation of tumor suppressor genes.
These mutations disrupt normal cell cycle control and promote uncontrolled growth.
Hormonal Action
Certain hormones (e.g. estrogen, testosterone) can promote growth of hormone-sensitive tumors like breast or prostate cancer.
Hormones can stimulate proliferation of cells, increasing the chance of mutation.
Co-Carcinogens
These are substances that enhance the effect of carcinogens but aren’t carcinogenic on their own.
They act by promoting inflammation or interfering with DNA repair mechanisms.
Tumor Promoter Effects
Tumor promoters are agents that stimulate cell proliferation after the initial genetic mutation.
They do not cause DNA damage themselves but enhance tumor development by increasing the proliferation of mutated cells.
Proto-oncogenes:
Normal genes involved in cell growth and division.
When mutated, they become oncogenes.
Oncogenes:
Mutated proto-oncogenes that promote uncontrolled cell division and survival.
Gain-of-function mutations — only one allele needs to be mutated for effect.
Tumor Suppressor Genes:
Genes that inhibit cell growth and promote DNA repair or apoptosis.
Loss-of-function in both alleles leads to cancer progression (e.g., TP53, RB).
Types of Cancer chemotherapy
Curative Chemotherapy
Aimed at complete eradication of the cancer.
Most effective in cancers with high chemosensitivity.
Examples:
Testicular cancer
Lymphomas (e.g., Hodgkin and non-Hodgkin)
Leukaemias (especially acute types)
Adjuvant Chemotherapy
Given after surgery or radiation to eliminate micrometastases and reduce relapse risk.
Improves long-term survival.
Examples:
Breast cancer
Colon and rectal cancers
Multimodal (Combined-Modality) Therapy
Combines chemotherapy with surgery and/or radiation.
Used when cancer requires different treatment strategies for local and systemic control.
Examples:
Head and neck tumors
Lung cancer
Cervical and esophageal cancer
Sarcomas
Pediatric solid tumors
Emerging/Advanced Approaches
Involves novel therapies alongside traditional chemo:
Genetic therapy – targeting specific mutations.
Immunotherapy – manipulating immune response to attack cancer (e.g. checkpoint inhibitors).
Angiogenesis inhibition – blocking blood supply to tumors (e.g., bevacizumab).
Hematopoiesis stimulation – using agents like G-CSF to support bone marrow during chemo.
Uses of chemotherapeutic agents
Cytotoxic Anti-Tumor Therapy
Used to kill or inhibit the proliferation of cancer cells.
Examples: Methotrexate, Cyclophosphamide, Doxorubicin.
Immunosuppressive Therapy
Used to suppress abnormal immune responses in:
Autoimmune diseases (e.g., Rheumatoid Arthritis, Lupus)
Organ transplantation (to prevent rejection)
Examples: Azathioprine, Methotrexate, Cyclosporine.
Treatment of Sickle Cell Anemia
Some agents like Hydroxyurea increase fetal hemoglobin (HbF) levels, reducing sickling.
Psoriasis
Cytotoxic/immunosuppressive drugs reduce abnormal skin cell proliferation.
Example: Methotrexate, Cyclosporine.
Anti-Infective Chemotherapy
Includes antibiotics, antivirals, antifungals, and antiparasitics.
Targets pathogens selectively without harming host cells.
MOA of chemotherapeutic agents (6)
DNA Interaction & Damage
Direct interaction with DNA: Causes cross-linking, strand breaks, or interference with replication.
Example: Alkylating agents like cyclophosphamide.
Irreparable DNA damage: Triggers apoptosis in rapidly dividing cells.
Example: Cisplatin.
Inhibition of Genetic Material Synthesis
Blocks DNA or RNA synthesis, especially in dividing cells.
Example: Antimetabolites like methotrexate (inhibits dihydrofolate reductase) or 5-FU.
Anti-Proliferative Action
Targets mitosis or cell division machinery, halting proliferation.
Example: Paclitaxel (stabilizes microtubules), vincristine (prevents microtubule formation).
Immune Modulation
Enhances tumor-killing immune cells:
Example: Interleukin-2 (IL-2) stimulates proliferation of cytotoxic T cells and NK cells.
Kinase Inhibition
Inhibits tyrosine kinases that send growth signals in cancer cells.
Example: Imatinib – a tyrosine kinase inhibitor used in CML (targets BCR-ABL fusion protein).
Tyrosine kinases are enzymes that signal cell growth and survival.
Monoclonal Antibodies
Specifically target tumor antigens, leading to direct killing or immune-mediated destruction.
Examples:
Rituximab – targets CD20 on B-cells
Trastuzumab – targets HER2/neu in breast cancer
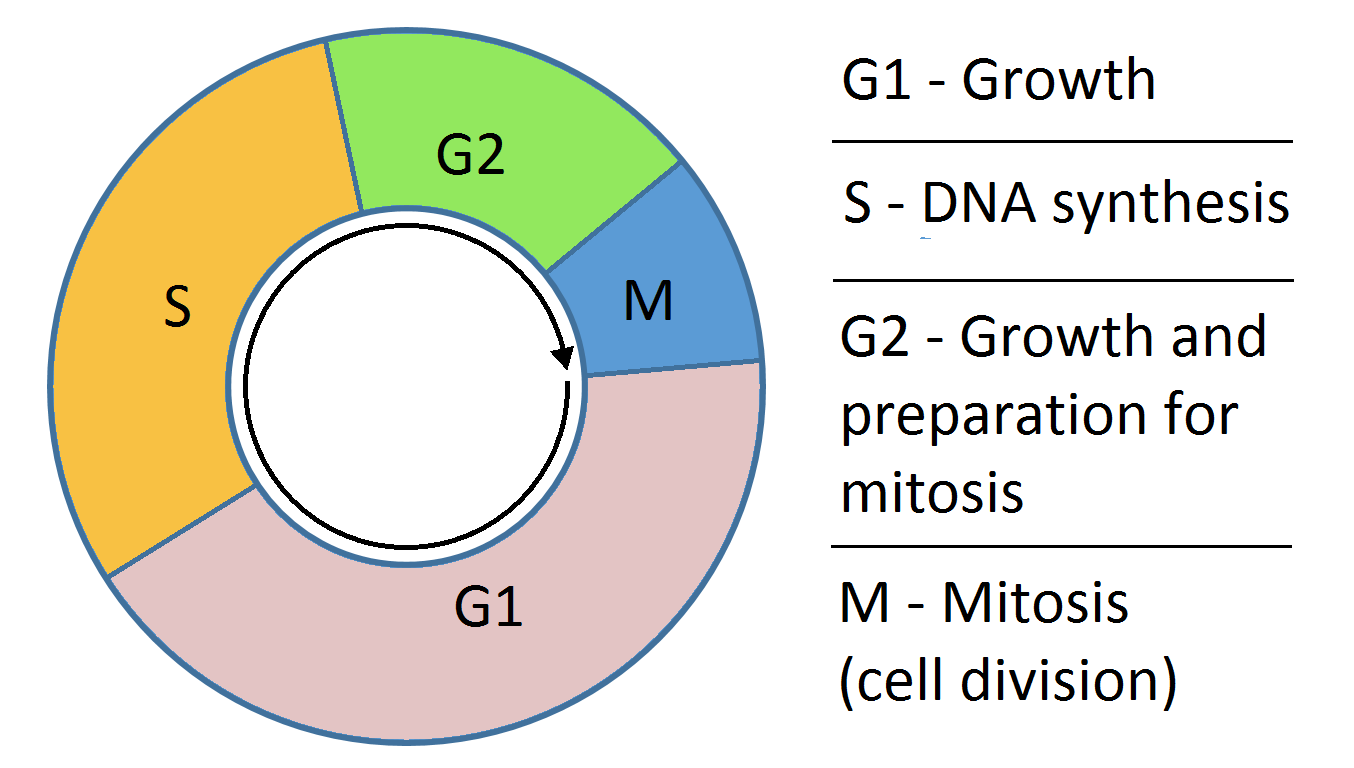
cell cycle
Cell cycle
• S phase - DNA synthesis
• G 2 phase - pre-mitotic interval
• M phase - mitosis
• G 1 phase - period between mitosis and DNA
synthesis
• Go phase - resting phase
Positive regulators of the cell cycle
• Cyclins
• Cyclic dependent kinases
Negative regulators of cell cycle
• P 53 protein
• Rb protein
• Cdk inhibitors
Chemotherapeutic regimen
• Combination therapy - synergism
• Drug interaction and toxicity
• Use drug with non overlapping mechanism of resistance and toxicity
• Maximum dose and dose interval
Survival tumor cells is mainly due to
• Loss of p53 suppressor oncogene, loss of apoptosis
• Over expression of bcl-2 oncogene that causes cell proliferation
Relationship of anti tumor drugs to cell cycle
Drug Class | Phase of Cell Cycle Affected | Examples | Mechanism |
|---|---|---|---|
Phase Non-Specific | Active in all phases including G0 | Alkylating agents, Nitrosoureas, Antibiotics (e.g. doxorubicin), Procarbazine, Cisplatin, Dacarbazine | Damage DNA regardless of the cell’s position in the cycle |
S Phase Specific | DNA synthesis | Cytosine arabinoside, Hydroxyurea | Inhibit DNA synthesis or cause faulty DNA incorporation |
S Phase Specific (Self-limiting) | S phase but with limited duration of activity | Methotrexate, 6-Mercaptopurine | Inhibit nucleotide synthesis → interfere with DNA replication |
M Phase Specific | Mitosis | Vincristine, Vinblastine, Paclitaxel | Inhibit mitotic spindle formation (microtubule inhibitors) |
🔬 Key Points
S phase is highly sensitive to drugs because of active DNA synthesis — thus toxicity (e.g., bone marrow suppression) is often greatest here.
M phase drugs (like vinca alkaloids and taxanes) prevent proper chromosome segregation → cell division arrest.
Phase non-specific drugs are useful for killing both dividing and resting cancer cells.
GIVE EXAMPLES OF CELL CYCLE NON SPECIFIC CANCER DRUGS
• Alkylating agents -
Nitrogen mustards -
mechlorethamine
cyclophosphamide,
melphalan,
chlorambucil
• Ethylenimines -
triethylenethiophosphoramide(Thio-TEPA)
• Methylhydrazine derivatives-
procarbazine
• Triazenes
dacarbazine
• Nitrosoureas -
carmustine,
bendamustine
• Platinum coordination complexes -
cisplatin
carboplatin
oxaliplatin
• Antibiotics - dactinomycin, daunorubicin, doxorubicin, plicamycin, mitomycin
GIVE EXAMPLES OF CELL CYCLE SPECIFIC CANCER DRUGS
These drugs act only when cells are in specific phases of the cell cycle, so they are most effective against rapidly dividing cells.
Drug Class | Examples | Phase of Cell Cycle Affected | Mechanism of Action |
|---|---|---|---|
Antimetabolites | Cytarabine, 5-Fluorouracil (5-FU), 6-Mercaptopurine (6-MP) | S phase | Inhibit DNA synthesis by mimicking normal nucleotides |
Peptide Antibiotics | Bleomycin | G2 phase | Causes oxidative damage to DNA, mainly before mitosis |
Podophyllotoxins | Etoposide, Teniposide | G2/phase | Inhibit topoisomerase II → DNA strand breaks |
Plant Alkaloids | Vincristine, Vinblastine, Vinorelbine | M phase | Inhibit microtubule assembly → block mitotic spindle |
Taxanes | Paclitaxel Docetaxel Cabazitaxel | M phase | Stabilizes microtubules → prevents their disassembly |
💡 High-Yield Tip:
These drugs won’t work well on non-dividing (G0) cells.
Combining cell cycle-specific with non-specific agents helps target a broader range of tumor cells.
Alkylating agents
• Cyclophosphamide
• Meclorethamine
• Melphalan
• Chlorambucil
• Ifosfamide
• Thiotepa
• Busulpan
MOA of alkylating agents
• Transfer alkyl group to various cellular constituents
• Leads to cell death
• Resistance by repairing of DNA
Nitrosoureas examples
• BCNU - Carmustine
• CCNU - Lomustine
• Methyl - CCNU -semustine
• Methyl - CCNU -semustine
Other than alkylating agents other anticancer drugs
• Antimetabolites
• Antibiotic and
• Vinca alkaloid
Anti tumor antibiotics
• Actinomycin D
• Dactinomycin
• Daunorubicin, Doxorubicin, Idarubicin
• Bleomycins
What are antimetabolites
Antimetabolites are drugs that interfere with the metabolism of rapidly dividing cells, especially cancer cells.
Cancer cells grow and divide faster than normal cells, so they rely more heavily on certain metabolic pathways — this makes them more vulnerable to these drugs.
Antimetabolite drugs are classified as
Folic acid analogs
Purine analogs
Pyrimidine analogs
MOA
• Act on nucleotide and nucleic acid synthesis
🔬 Mechanism of Action of Antimetabolites
Antimetabolites are structural analogs of purines, pyrimidines, or folic acid — all of which are essential for DNA and RNA synthesis. They work in three main ways:
1. Inhibit synthesis of nucleotides
They block enzymes that are essential for making purine or pyrimidine nucleotides (the building blocks of DNA/RNA).
Examples:
Methotrexate inhibits dihydrofolate reductase (DHFR) → ↓ tetrahydrofolate → ↓ purine & thymidylate synthesis
5-Fluorouracil (5-FU) inhibits thymidylate synthase → ↓ dTMP → impaired DNA synthesis
2. Mimic nucleotides and get incorporated into DNA/RNA
These drugs look like real nucleotides, so cells mistakenly incorporate them into DNA or RNA during synthesis.
This leads to:
Faulty DNA/RNA
Chain termination
Mutation or apoptosis
Examples:
6-mercaptopurine and 6-thioguanine → incorporated into DNA/RNA
Cytarabine → incorporated into DNA → chain termination
3. Trap enzymes in inactive complexes
Some antimetabolites bind to enzymes in a way that traps them in inactive or toxic forms, halting the reaction and damaging the cell.
Example:
5-FU forms a complex with thymidylate synthase + folate → irreversible inhibition
🎯 Net Effect:
↓ DNA/RNA synthesis
↓ Cell replication
☠ Death of rapidly dividing cells (like cancer cells)
Folic acid analogues
• Methotrexate
• Pemetrexed
• Raltitrexed
• Lometrexol
• Trimetrexate
• Pralatrexate
Methotrexate - MOA
Structurally similar to folic acid, it competitively inhibits the enzyme dihydrofolate reductase (DHFR).
DHFR normally converts dihydrofolate (DHF) to tetrahydrofolate (THF), which is essential for:
Thymidylate synthesis (→ DNA)
Purine nucleotide synthesis (→ DNA and RNA)
Amino acid synthesis (e.g., serine, methionine)
Result: Impaired DNA, RNA, and protein synthesis, especially in rapidly dividing cells.
Key Binding Property:
Methotrexate binds extremely tightly to DHFR, particularly at pH 6, with little to no dissociation, making it a potent inhibitor.
Methotrexate Resistance Mechanisms
↓ Drug Transport into the Cell
Due to decreased expression or mutation of the folate transporter (RFC1), which normally allows methotrexate to enter cells.
Altered Dihydrofolate Reductase (DHFR)
Mutations in the DHFR enzyme reduce its binding affinity for methotrexate.
↑ DHFR Production
Gene amplification leads to overproduction of DHFR enzyme.
This overwhelms the inhibitory effect of methotrexate.
Results in increased DHFR mRNA and protein levels.
↓ Polyglutamate Formation
Methotrexate is normally polyglutamated inside cells to enhance retention and activity.
Decreased polyglutamate formation → less drug retained in the cell → reduced efficacy.
PK of methotrexate
• PO or IV or IM or INTRATHECALLY
Well distributed into body cavities
50% PPB
metabolised partially
• 90% of oral dose excreted in urine within 12 hours
RETAINED AS POLYGLUTAMATE FOR WEEKS IN THE KIDNEY AND MONTHS IN THE LIVER
• Serum levels proportional to dose as long as serum levels and hydration status are adequate
Toxicity of methotrexate
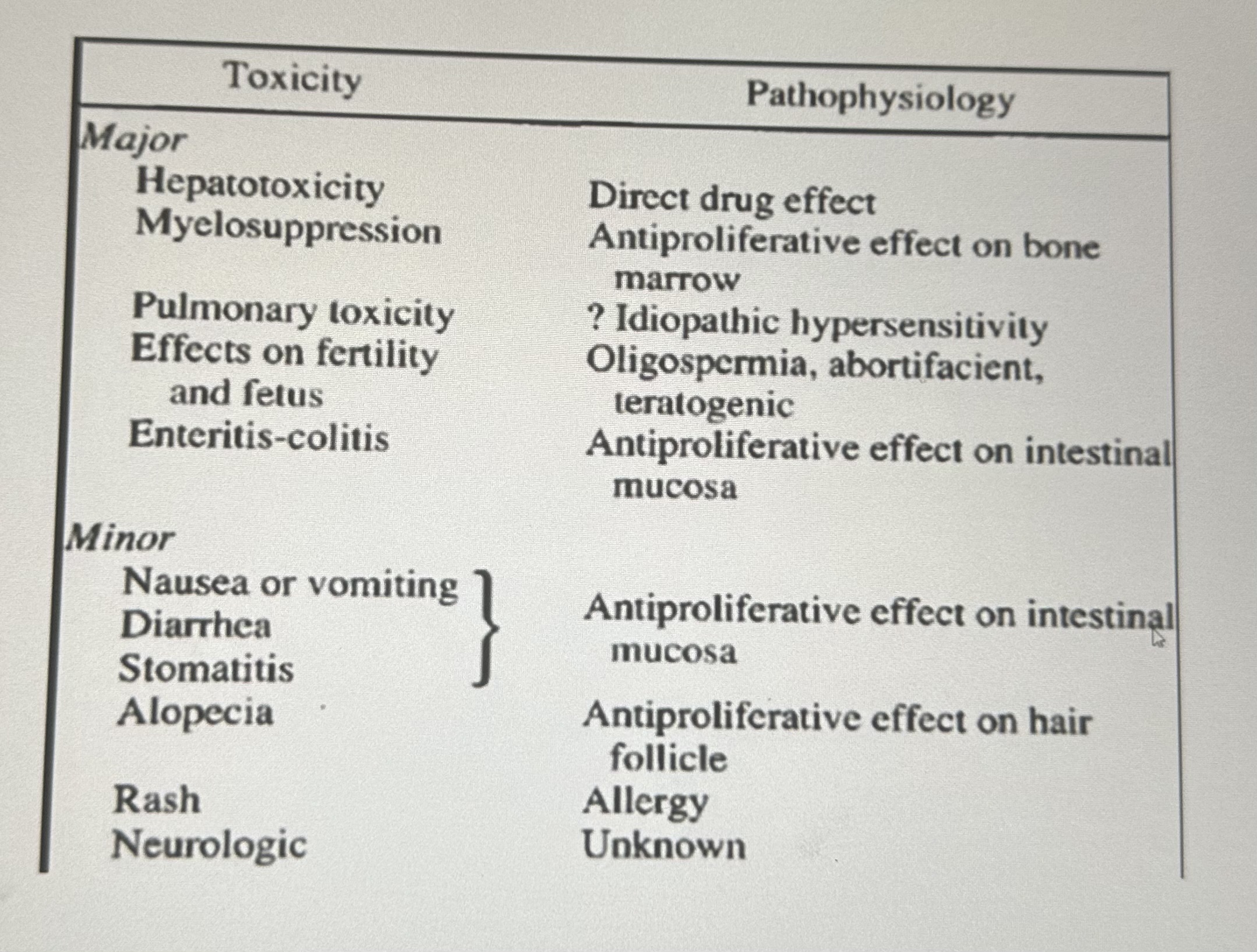
ADRS
* BM: Myelosupression,thrombocytopenia
* Liver: Fibrosis, cirrhosis
* GIT: Nausea,vomiting,diarrhoea,mucositis, stomatitis,desquamation
* Skin: Erythema,rash,urticaria,alopecia, dermatitis
* Resp:Interstitial pneumonitis
* CNS: Meningismus,headache,seizure, coma
* Genital:Defective oogenesis,spermatogenesis
* Teratogenicity and abortions
* High dose:Nephrotoxicity
Use of methotrexate in neoplasia
• Choriocarcinoma
• ALL in children
• Meningeal leukaemia, lymphoma
• Burkitt's lymphoma,NHL, Ca breast, head & neck
• AML
• HDM-L
Osteosarcoma
Non neoplastic indications of methotrexate
* Psoriasis
* Refractory RA
* Steroid resistant asthma
* Crohn's disease
* Wegener's granulomatosis
* Glomerulonephritis
* Dermatomyositis
* Immunosuppressive agent
* Abortifacient
Toxicity of methotrexate to normal cells is reduced by
Leucovorin rescue- administration of :
N10-formyl-tetrahydrofolic acid / folinic acid / citrovorum factor / leucovorin.
IT IS CONVERTED TO 5 , 10 methylene tetra hydrofolate→ bypassing the enzyme
*IT DOES NOT PREVENT NEUROTOXICITY
Alkalanisation of urine can also reduce methotrexate toxicity.
Administration of GLUCARPIDASE in extreme cases. glucarpidase is a methotrexate cleaving agent.
Methotrexate should not be administered with
NSAIDs like aspirin
Penicillins
cephalosporins
THEY MAY DECREASE RENAL EXCRETION OF METHOTREXATE AND RESULT IN TOXICITY.
PE
Purine antagonists
- 6-thiopurines
6-Mercaptopurine
- 6-Thioguanine
•
Purine analogues / antagonists
Classic purine analogues
• Thiopurines
6 Mercaptopurine
6 Thioguanine
others - Fancy Cool Chemo People Need Purines
• Pentostatin
• Fludarabine PO4
• Cladribine
• Clofarabine
' nelarabine
Mercaptopurine MOA
• Metabolised by hypoxanthine guanine phosphoribosyl transferase (HGPRT) to the nucleotide form 6- thioinosinic acid (6-Tl) / Thioinosine monophosphate (TIMP)
TIMP interferes with de novo purine synthesis by inhibiting a number of enzymes -
It inhibits amidophosphoribosyltransferase (also called PRPP amidotransferase), which is the rate-limiting enzyme in the purine synthesis pathway.
It also interferes with the conversion of IMP to AMP and GMP by inhibiting:
IMP dehydrogenase
Adenylosuccinate synthetase
also gets incorporated into DNA and RNA, leading to:
Inhibition of DNA and RNA synthesis
Cytotoxicity in rapidly dividing cells
• 6-thioguanalic acid and 6- methylmercaptopurineribotide are also formed from 6-MP may also be active
MOA of Thioguanine
• Inhibits several enzymes in the purine nucleotide pathway
• May cause metabolic lesions
- inhibition of purine nucleotide interconversion
- decreases intracellular guanine nucleotide thus inhibition of glycoprotein synthesis
- interference with formation of DNA and RNA
- incorporation of purine thiols in DNA and RNA
- synergy of 6- MP with cytarabine
PK of 6- mecaptopurine and 6-thioguanine
• 6-MP and 6-TG are both given PO
• Excretion is mainly in urine
• 6-MP is metabolised by xanthine oxidase and converted to an inactive metabolite 6-thiouric acid (oxidation)
• 6-TG is metabolised by the same enzyme following deamination
Allopurinol use in chemotherapy
ALLOPURINOL IS USED IN CHEMOTHERAPY IN HAEMATOLOGICAL CANCERS TO PREVENT TUMOR LYSIS INDUCED HYPERURICEMIA.
• Allopurinol prevents hyperuricaemia by blocking purine oxidation(XANTHINE OXIDASE INHIBITOR) allowing excretion of cellular purines that are relatively more soluble that uric acid
•OTHER - Prevents nephrolithiasis and acute gout
WHAT SHOULD BE DONE WHEN CO ADMINISTERING BOTH ALLOPURINOL AND 6- MECAPTOPURINE?
• Concomitant use of 6-MP and allopurinol results in excessive toxicity of 6-MP thus dose reduction of 6-MP by 25-30% or 1/4th of the original dose
• This does not occur with 6-TG
HOW DOES RESISTANCE TO 6-MP OR 6-TG DEVELOP?
• Decrease in hypoxanthine-purine phosphoribosyl transferase activity
~ HGPRTase is required to convert these prodrugs into their active nucleotide forms.
Without activation, the drugs have no chemotherapeutic effect.
Uses of 6-MP and 6-TG
• Childhood acute leukaemias.
• Analogue of 6-MP - azathioprine is used as immunosuppressant
Fludarabine phosphate MOA
• Interferes with DNA synthesis through inhibition of DNA polymerase and ribonucleotide reductase.
Administration of fludarabine phosphate
Given parenterally and excreted in urine
Clinical use of fludarabine phosphate
Used in the treatment of lymphoproliferative disorders
DOC - CLL
ADRS of fludarabine phosphate
• Dose limiting myelosuppression
Cladribine - MOA
• Cause DNA strand breaks by interfering with DNA repair
• Use in treatment of hairy cell leukemia
• Myelosuppresant
Cladribine - clinical use
Use in treatment of hairy cell leukemia - DOC
• Myelosuppresant
Pyrimidine antagonists
• Fluorouracil
• Capecitabine
• Cytarabine
• Gemcitabine
Fluorouracil MOA
5 - FU is a prodrug.
5-FU is converted in the body into active nucleotide forms by deoxyuridine monophosphate.
One form, FdUMP( 5 fluoro-2-deoxycytidine- 5'phosphate), FdUMP competes with dUMP (the natural substrate) for binding to thymidylate synthase and binds to and inhibits thymidylate synthase, blocking DNA synthesis.
Another form, FUTP(Fluorouridine triphosphate), gets incorporated into RNA, disrupting RNA function.
These effects lead to cell death → that’s how 5-FU is cytotoxic.
Fluorouracil - PK
ADMIN -
IV
Topically in the case of skin cancer
Erratic bioavailability PO
DISTRIBUTION -
The drug penetrates will into tissues including CNS
METABOLISM -
Rapidly metabolised in the liver, kidney, lungs and converted to fluoro -beta - alanine → EXCRETED in urine.
Short metabolic half-life
— can increase the rate of catabolism and decrease bioavailability of 5-FU
elevated levels of Dihydropyrimidine dehydrogenase .
Varies from individual to individual
Pts with Dihydropyrimidine dehydrogenase deificiency may experience severe toxicity-
pancytopenia
mucositis
life threatening diarrhea
5-FU clinical use
Topical for skin cancer
Systemically for adenocarcinoma
ADRS of 5-FU
Myelosuppression
mucositis
Capecitabine is a
• Fluoropyrimidine carbamate prodrug converted to 5-FU
Capecitabine - MOA
• Given orally
It is hydrolysed to 5 -FU as the last enzymatic step. It is catalysed by thymidine phosphorylase an enzyme that is primarily concentrated primarily in tumors
Capecitabine clinical use
Colorectal cancer
Metastatic breast cancer
Cytarabine is specific to what phase in the cell cycle
• S- phase specific antimetabolite
• Metabolite inhibits DNA polymerase
Cytarabine PK
• Admin - IV
NOT ORALLY - due to deamination to noncytotoxic ara-U by cytidine deaminase in the intestinal mucosa and liver.
Distributes throughout the body EXCEPT THE CNS → THEREFORE IT MAY BE INJECTED INTRATHECALLY
Metabolised - undergoes extensiive oxidative deamination in the body to
ara-U - pharmacologically inactive.
Excretion - Both cytarabine and ara-U are excreted in urine.
Cytarabine clinical use
AML
Gemcitabine clinical use
treatment of non-small cell lung cancer
pancreatic cancer
• Myelosuppresant
Gemcitabine PK
IV administration
is deaminated to difluorodeoxyuridine - noncytotoxic
excreted in urine.
Vinca alkaloids are derived from
• Part of the natural product cancer chemotherapy drugs
• Derived from the Madagascar periwinkle plant, Catharanthus roseus (formerly called Vinca rosea).
• Include:
i. Vincristine ii. Vinblastine iii. Vinorelbine
vinca alkaloids includes
Include:
i. Vincristine
ii. Vinblastine
iii. Vinorelbine
MOA
MITOTIC SPINDLE INHIBITORS
• Inhibit tubulin polymerization→ disrupts assembly of microtubules, an important part of the cytoskeleton and the mitotic spindle.
• Results in mitotic arrest in metaphase→halts cell division, leading to cell death.
Microtubules are found in high concentrations in the brain - disruption causes neurotoxicity
Are vinca alkaloids cell cycle specific
specific to the M phase
inhibit mitotic spindle formation → preventing cell division
PK OF VINCA ALKALOIDS
Aministration - IV
VINCA ALKALOIDS SHOULD NOT BE ADMINISTERED INTRATHECALLY → IN CAN RESULT IN DEATH.
Extensively metabolized in the liver
Metabolites are excreted in bile
< 15% is going in the urine unchanged
Dose adjustment required in patients with hepatic dysfunction
Half lives:
Vincristine= 20 hrs
Vinblastine= 23 hrs
Vinorelbine=24 hrs
Vinblastine
Is given intravenously
• Avoid subcutaneous extravasation → painful irritation and ulceration.
Therapeutic uses of vinblastin
i. Testicular tumors (administered with bleomycin and cisplastin)
ii. Hodgkin's lymphoma
iii. Kaposi sarcoma
iv. Neuroblastoma
v. Langerhans cell histiocytosis
vi. Carcinoma of the breast
vii. Choriocarcinoma.
Clinical toxicities
• Leukopenia- nadir (lowest WBC count) within 7-10 days, with recovery in another 7 days.
VBL IS A POTENT MYELOSUPPRESSANT.
• Mild neurological manifestations.
• Nausea, vomiting, anorexia & diarrhea.
• Alopecia, stomatitis & dermatitis.
• Extravasation → cellulitis.
Vincristine
ADMINISTRATION AND DOSAGE
Is given IV
dose - • Dose : 2mg/m2 in children : 1.4mg/m2 in adults
• Better tolerated by children than adults, who may experience severe, progressive neurological toxicity.
• Given at weekly intervals.
Therapeutic uses
• Childhood leukemia - ALL
• Pediatric solid tumors- Wilms tumor, neuroblastoma & rhabdomyosarcoma
Hodgkin’s lymphoma
• Non Hodgkin’s lymphoma
Clinical toxicities of vincristine
• Mostly neurological- sensory & motor disturbances → NEUROTOXIC - PERIPHERAL NEUROPATHY
• Severe constipation
• Alopecia in 20% (reversible without cessation of therapy)
• Thrombocytopenia, anemia.
• Myelosuppression is much less than that caused by Vinblastine. (VINOCRISTINE IS BONE MARROW SPARING)
• Extravasation → cellulitis and phlebitis
Vinorelbine administration and dosage
Administered in normal saline as an iv infusion over 10 minutes
• Dose: 25- 30 mg/m2 weekly
Indications of vinorelbine
Useful in:
i. Non-small cell lung cancer
ii. Breast cancer
Clinical toxicities of vinorelbine
Has an intermediate toxicity profile
• Primary toxicity is granulocytopenia
• Allergic reactions
• Mild changes in liver enzymes
Advantage of vinorelbine compared to other vinca alkaloids
Less neurotoxicity than other vinca alkaloids
Antitumor antibiotics are produced by
• Are produced by Streptomyces strains
• Bind to DNA within tumor cells and interfere with replication
MOA of antitumor antibiotics
• Bind to DNA within tumor cells and interfere with replication
Antitumor antibiotics includes
Include:
a. Anthracyclines
b. Bleomycin
c. Mitomycin
d. Mitoxantrone
Anthracyclines are isolated from
• Are isolated from Streptomyces peucetius var caesius.
• Examples:
a. Doxorubicin
b. Daunorubicin
c. Idarubicin
d. Epirubicin
Anthracyclines include
a. Doxorubicin
b. Daunorubicin
c. Idarubicin
d. Epirubicin
Are antitumor antibiotics cell cycle specific or non specific
ALL CELL CYCLE NON SPECIFIC EXCEPT BLEOMYCIN
Pk of anthracyclines
• Given IV
• Extensive liver metabolism to an active hydroxylated metabolite & an inactive aglycone metabolite.
• Up to 50% is eliminated in the faeces.
• Dose reduction required in liver dysfunction.
• Usually given every 3 weeks.
MOA of cytotoxic action of antitumor antibiotics (4)
1. Inhibition of topoisomerase lI
2. DNA intercalation, blocking synthesis of DNA & RNA.
3. Generation of semiquinone- and oxygen free radicals
4. Binding to cellular membranes to alter ion transport.
Daunorubicin use
• First agent in the class to be isolated
• Use- treatment of acute myeloid leukemia (AML).
Idaribicin
IDARUBICIN
• Semisynthetic analog of Daunorubicin
• More active than Daunorubicin in achieving remission and improving survival in AML.
DARUBICIN
• Semisynthetic analog of Daunorubicin
• More active than Daunorubicin in achieving remission and improving survival in AML.
DOXORUBICIN USE
• Solid tumors- breast, endometrium, ovary, testicle, thyroid, stomach, bladder, liver, and lung.
• Hematologic malignancies- acute lymphoblastic leukemia, multiple myeloma, Hodgkin's and non-Hodgkin's lymphomas.
• Childhood cancers- neuroblastoma, Ewing's sarcoma, osteosarcoma, rhabdomyosarcoma.
ADRs of anthracycline antibiotics
1. Myelosuppression- Dose limiting.
Neutropenia >>>>thrombocytopenia.
2. Mucositis- may also be dose-limiting.
3. "Radiation recall reaction" - erythema & desquamation of the skin at sites of prior radiation.
4. Cardiotoxicity: → Due to free oxygen radical damage to the myocardium
a. Acute- First 2-3 days & transient. Arrhythmias, pericarditis, myocarditis.
b. Chronic
- Dilated cardiomyopathy with heart failure
- Seen with cumulative dose >500-550mg/m2.
Cardiotoxicity of anthracycline antibiotics can be reduced by use of
Dexrazoxane → free radical scavenger
MITOMYCIN is isolated from
• Antibiotic isolated from Streptomyces caespitosus.
Mechanism of cytotoxic action of mitomycin
MITOMYCIN ACTS AS AN ALKYLATING AGENT
• Activated through enzyme-mediated reduction → generates an alkylating agent → cross-links DNA.
• Hypoxic tumor stem cells of solid tumors thus more sensitive to its cytotoxic effect as they exist in an environment conducive to reductive reactions.
Clinical uses of mitomycin
• Best drug to use in combination with radiation therapy to attack hypoxic tumor cells.
• Squamous cell cancer of the anus & cervix.
Breast, gastric & pancreatic cancer.
Intravesical treatment of superficial bladder cancer (no systemic toxicity).
ADRS of mitomycin
• Hemolytic uremic syndrome ~ rare
• Interstitial pneumonitis
• Myelosuppression
• Mucositis
GIT symptoms
BLEOMYCIN MOA
• Structure- Is a small peptide containing a DNA-binding & iron-binding domain.
Causes DNA strand breakage by oxidative process and free radical formation.
Bleomycin forms a complex with Fe²⁺ (ferrous iron) and DNA.
This complex gets oxidized to Fe³⁺, releasing electrons.
The electrons react with O₂ to form superoxide and hydroxyl radicals (ROS).
These ROS attack DNA, breaking phosphodiester bonds → causes:
Strand breaks
Chromosomal damage
IS BLEOMYCIN CELL CYCLE SPECIFIC OR NON SPECIFIC
Is cell cycle specific
• Causes accumulation of cells in the G2 phase.
BLEOMYCIN PK
• Given SC, IM or IV.
instillation in the bladder for bladder ca.
Bleomycin is hydrolysed by bleomycin hydrolase to inactive metabolites. The enzyme is in low concentrations in the skin and lungs ~thus are major organs involved in toxicity.
• Excretion mainly via kidneys.
• Requires dose adjustment in renal failure.
BLEOMYCIN Clinical use
• Hodgkin's and non-Hodgkin's lymphomas,
• Germ cell tumors
• Head and neck cancer
• Squamous cell cancer of the skin, cervix & vulva
BLEOMYCIN ADRS
• Pneumonitis- dose-limiting. Risk increased with cumulative doses > 400 units.
• Pulmonary fibrosis
• Mucositis
• Alopecia
Hyperpigmentation of the hands
Hyperkeratosis
erythema
• Acute- allergy, fever, hypotension
myelosuppression is rare
Minimal myelosuppressive and immunosupressive
DACTINOMYCIN is also known as
actinomycin D
Crystalline antibiotic from streptomyces culture