Physics 3.5
Demonstrate understanding of Modern Physics - Internal, 3 credits
The Photoelectric Effect
A Photon is a packet of electromagnetic radiation that travels at the speed of light (3.00×10^8ms^{-1}). There are a number of properties of a photon:
Frequency (f) → how frequent the oscillations of the wave are
Intensity (I) → how many photons there are in a stream
Wavelength (\lambda) → how long each wave is, which determines where on the electromagnetic spectrum the photon is. The wave equation v=\lambda{f} describes how the velocity, frequency, and wavelength are linked.
Photons can be described as having Wave-Particle Duality, as they have properties of both waves and particles. They can be diffracted, refracted, and reflected like waves, but carry a discrete amount of energy, like a particle would. Photons have no mass.
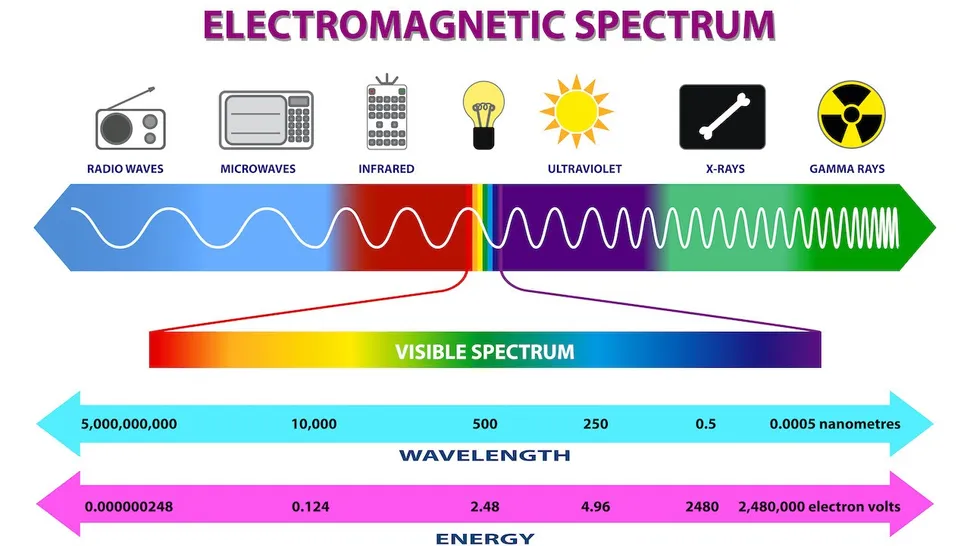
Each Colour photon has the same energy, frequency, and wavelength. Note that colour can be visible, but most wavelengths are not visible to the human eye.
The Photoelectric Effect describes a phenomenon that occurs in metallic materials when hit by a photon. If the photon has sufficient energy (called the work function, symbol \phi), it will transfer the energy to an electron, causing it to be removed from its atom. If just the work function is applied, the electron will be removed, but it will not leave the surface. Any additional energy will be transformed into kinetic energy, allowing the electron to move away. If less energy than the work function is applied, the surface will heat up, but no change to its electron will occur. The photoelectric effect only works in metallic substances, as non-metals hold the electrons too tightly for them to leave.
The photoelectric effect can be described using the equations:
E=hf, where E is the energy of a photon (in J), f the frequency (in Hz) and h is Planck’s constant: 6.63×10^{-34}Js.
E_{photon}=\phi+E_k, where \phi is the work function and E_k is the kinetic energy of the electron.
These equations combine to give hf=\phi+E_k.
Each metal has its own Work Function. The energy for each frequency can be graphed to find the work function (y-intercept), and the gradient will be Planck’s constant:
As photons and electrons have such a small amount of energy each, a unit of energy called the Electron Volt (eV) can be used instead of joules. 1.0eV=1.602×10^{-19}J.
A Photoelectric Cell (also called photovoltaic cell) can be used to generate a current using the photoelectric effect. There are three main components of a photoelectric cell:
The emitter → a piece of metal (curved towards the collector) that light shines on.
The collector → where the electrons are collected after emission.
These are housed in a glass vacuum chamber. This prevents any air molecules from interfering with the electrons after emission, or oxidising the emitter, which would reduce its efficiency.
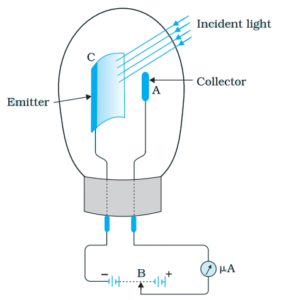
The kinetic energy of an emitted electron can be found using E_K=eV, where e is the charge on an electron 1.6×10^{-19}C and V is the voltage across the cell.
The Threshold Frequency is the frequency of light that has energy identical to the work function of the surface.
If the photon frequency is less than the threshold frequency, the electron remains bound to the surface and it will heat up.
If the photon frequency is equal to the threshold frequency, the electrons overcome the binding forces holding them to the surface, but remain on the surface.
If the photon frequency is greater than the threshold frequency, the electrons will gain kinetic energy and move away from the surface.
As the electrons are emitted, the emitter becomes positively charged and the collector becomes negatively charged. This creates a voltage across the cell. When connected to a variable power supply, the voltage across the photoelectric cell can be altered.
When no voltage is applied, electrons are emitted, but scattered so not all will reach the collector.
When a positive voltage is applied (positive collector and negative emitter), the electrons are attracted to the collector so there will be less scattered electrons and an increased current. The maximum possible current (when every emitted electron hits the collector) is called the Saturation Current.
When a negative voltage is applied (negative collector and positive emitter), the electrons are repelled by the collector, so the current will decrease. The voltage where no current will flow is called the Stopping Voltage.
When light of a higher intensity is used, more electrons will be emitted, so the saturation current will be higher. However, the stopping voltage will remain the same as each photon still has identical energy.
When light of a higher frequency is used, the saturation current will remain the same, as the same number of photons are hitting the emitter. However, due to the increased energy of the photons, a higher (negative) voltage is required to prevent the electrons (which have more kinetic energy) from hitting the collector. Therefore, the stopping voltage is more negative.
Bohr Model and Atomic Line Spectra
Many models of the atom have been proposed over the years, which have been continuously improved upon. Rutherford’s planetary model of the atom proposed what we are familiar with today: a dense, positively charged nucleus orbited by negatively charged electrons. However, there were two major flaws with this model:
This did not explain how the photoelectric effect could occur
The electrons would lose energy and spiral into the nucleus over time
Niels Bohr developed a new model of the atom. This still contained the dense, positively charged nucleus, but made three major changes to the orbiting electrons:
Electrons orbit is discrete, stable shells called Energy Levels
Each energy level permits a fixed amount of energy per electron
Electrons must gain energy to move to a higher energy level or emit energy to move to a lower energy level. This energy is in the form of a Photon.
These predictions were proved experimentally for the hydrogen atom, but interactions between electrons made it difficult to prove for other atoms.
The lowest energy level is closests to the nucleus and is also called the Ground State. Each energy level is numbered according to its distance from the nucleus. The ground state is described as n=1, and the rest are numbered accordingly (n=2, n=3 etc). These levels theoretically continue infinitely.
When an electron gains energy, it is described as being excited. When it is excited so that it is no longer bound to the atom, this energy is called the Ionisation Energy Level. At this point, the electron has no energy, as it has all been used to overcome the attractive force between itself and the nucleus. This means that the energy of electrons in lower energy levels is negative, as an electron must lose energy as it moves down. The amount of energy an electron has in each energy level can be found using E_n=\frac{-chR}{n²}, where n is the energy level of the electron, c is the speed of light, h is Planck’s constant and R is the Rydberg constant (1.1×10^7m^{-1}). When an electron transitions between energy levels, the amount of energy in the photon that was absorbed or emitted can be found using \Delta{E}=E_f-E_i, as energy must be conserved.
The energy of a photon is dependent on its frequency. The frequency and wavelength of a photon can be found using E=hf and v=f\lambda, where v=c=3.00×10^8ms^{-1}.
Atomic Line Spectra come in two forms: emission spectra and absorption spectra. An emission spectrum is created when white light passes through a gaseous element with a high-voltage current. The gas will emit light in specific wavelengths, which can be separated using a prism. This spectrum will be mostly dark, with brightly-coloured bands of light at specific wavelengths. The wavelengths correspond to the energy of photons emitted when electrons move to lower energy levels. Absorption spectra are created when white light passes through a cold gas. The light emitted can be passed through a prism to create a spectrum, which is mostly bright colours with small dark bands at specific wavelengths. These correspond to the wavelengths of photons absorbed when electrons move to higher energy levels. For an element, its emission and absorption spectra will be the inverse of each other.
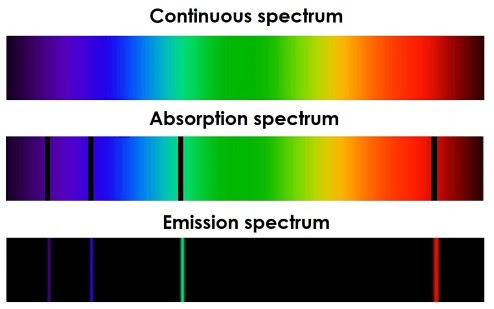
To calculate the wavelengths of hydrogen spectral lines, here are 5 spectral series that are used, which are numbered 1-5. These are:
Lyman Series → in the ultraviolet spectrum. This has a series number of 1.
Balmer Series → in the visible spectrum. This has a series number of 2.
Paschen Series → in the infrared spectrum. This has a series number of 3.
Brackett Series → in the infrared spectrum. This has a series number of 4.
Pfund Series → in the infrared spectrum. This has a series number of 5.
Spectral line series can be drawn, where absorption arrows point up and emission arrows point down. This represents the loss/gain of energy. The lines get closer together as the frequency increases. The length of the arrows represents the energy change that occurred in the transition.
The Rydberg formula is used to determine the wavelength of the spectral bands. It is: \frac{1}{\lambda}=R(\frac{1}{S²}-\frac{1}{L²}), where R is the Rydberg constant, S is the series number and L is the line number. The frequency of the photon can then be found using v=c=f\lambda.
To find the energy absorbed or emitted in a change of energy level, E=hf=\frac{hc}{\lambda} is used.
Nuclear Reactions
Every atom has two important quantities: the Atomic Number and Mass Number. The atomic number is the number of protons in the nucleus of the atom. The mass number is the total number of nucleons in the nucleus. Nucleons are protons and neutrons. Therefore, the number of neutrons can be found by subtracting the atomic number from the mass number.
Isotopes are atoms with the same atomic number but different mass numbers. For example, Cl-37 has 20 neutrons and Cl-35 has 18 neutrons, but they are both still chlorine.
As the nucleus contains many positively charged protons, it is expected that the electrostatic forces would repel these particles and push the nucleus apart. However, a more powerful force called the Strong Nuclear Force holds the nucleus together despite repulsive forces between protons. This is because protons and neutrons are made up of subnucleonic particles called Quarks, which are bonded together by the strong nuclear force (also called the strong interaction). This force acts over very short distances, which holds the nucleus together.
Radiation is emitted from the nucleus of an atom during nuclear reactions. The three main types are:
Alpha Particles (\alpha): These are the largest and slowest-moving type of radiation. An alpha particle contains two protons and two neutrons, the same as a helium nucleus. It has the symbol ^4_2\alpha.
Beta Particles (\beta): Beta particles are formed when a neutron breaks down into a proton, releasing some energy and negative charge as a high-speed electron, called a beta particle. Beta particles have the symbol ^0_{-1}\beta. Positrons are the same as beta particles, but have a positive charge, so have the symbol ^0_{+1}\beta.
Gamma Rays (\gamma): Gamma rays are an emission of electromagnetic radiation from the nucleus.
In a nuclear reaction, if nucleons are to break away from the nucleus, energy must be added as to overcome the strong nuclear force. This indicates that nucleons have more energy when they are free than they do when in the nucleus. In addition to the difference in energy, there is a difference in mass. The total mass of free nucleons is greater than the mass of the nucleus. This is accounted for in Einstein’s Theory of Special Relativity. Einstein proposed that mass and energy are equivalent as in the equation E=mc² . The rest mass of a particle is its mass when stationary, as if it was moving its mass would change due to this Mass-Energy Equivalence.
During a nuclear reaction, four quantities must be conserved:
Mass number
Atomic number
Momentum
Mass-energy
The difference in mass between a nucleus and its constituent free nucleons is called the Mass Defect. When energy is added to the nucleus, it break apart and the energy is converted to a greater mass of the resulting nucleons. In the reverse process, energy is emitted when nucleons join to form a nucleus. This leads to a loss in mass.
Particle | Charge | Rest Mass |
Proton | +1 | 1.6726×10^{-27} kg |
Neutron | 0 | 1.6749×10^{-27} kg |
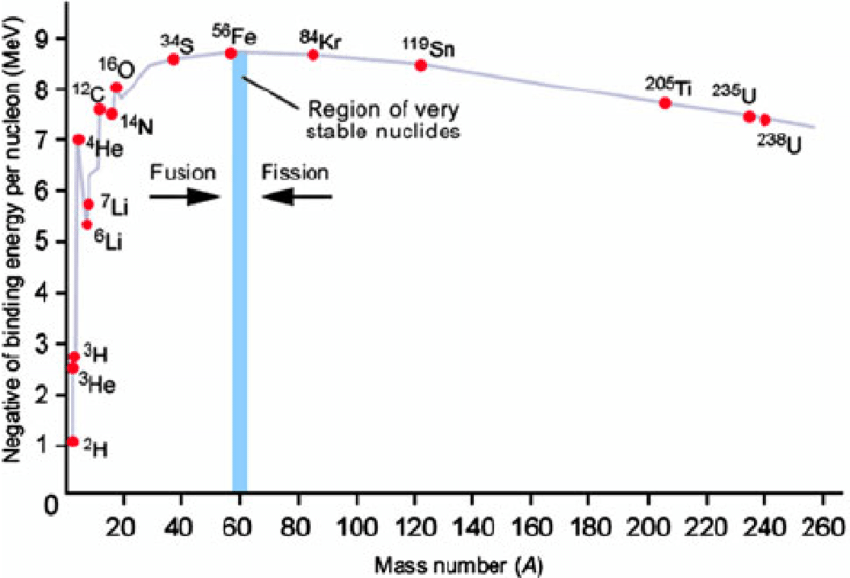
The Binding Energy is the total amount of energy required to break a nucleus into its constituent nucleons. To break one nucleon apart from the nucleus, the Binding Energy per Nucleon must be added. Nuclei with more binding energy per nucleon have less average mass per nucleon, so they are are stable.
Nuclear Fission is a type of nuclear reaction where the nucleus breaks apart into smaller, more stable nuclei. In unstable nuclei, fission can occur without external input. This is called spontaneous fission. In more stable elements, fission can be induced by adding a neutron which makes the nucleus unstable. This is called induced fission or stimulated fission. If more than one neutron is emitted during a fission reaction and it collides with more fissionable material, a chain reaction can begin which releases a very large amount of energy. Chain reactions can be dangerous due to the large amounts of energy they release.
Nuclear Fusion is a type of nuclear reaction where two or more nuclei combine to form a larger, more stable nucleus. Because the nucleons must overcome very strong repulsive forces, fusion requires large amount of energy, so fusion is typically found in stars.
Both fusion and fission produce more stable nuclei that have a higher binding energy per nucleon than the original nuclei, so they release energy in the process. This can be identified by a reduction in the average mass per nucleon (E=mc²). The most stable nucleus is Iron-56. Nuclear reactions tend to have daughter nuclei that are closer to Iron-56 than the parent nuclei.