Unit 1: Chemistry of Life
Biology - the scientific study of life
There is no biology without chemistry
Covalent bond - the sharing of a pair of the outermost shell valence electrons by two atoms
Molecules - two or more atoms held together by covalent bonds
Electronegativity - an atomś attraction for the electrons of a covalent bond (like gravity)
In a nonpolar covalent bond, the atoms share the electrons equally (H2).
In a polar covalent bond, one atom is more electronegative, and the atoms don’t share the electrons equally. This leads to partial charges (H2O).
Ionic bonds - result from the taking or losing of an electron which creates two oppositely charged ions that are attracted to each other.

Cation - positively charged ion.
Anion - negatively charged ion
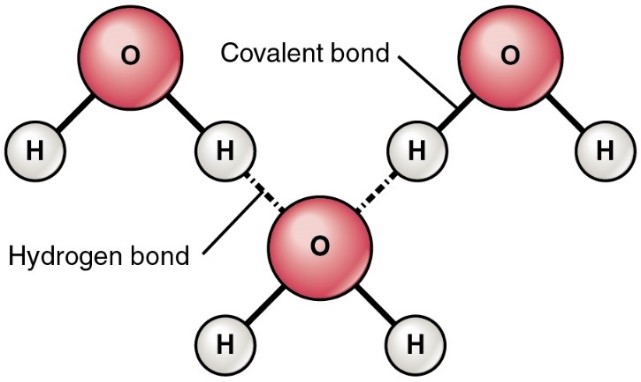
Hydrogen bonds - form when a hydrogen atom covalently bonded to one electronegative atom is also attracted to another electronegative atom. A hydrogen bond is considered a weak bond.
Properties of Water
The four emergent properties of water that contribute to Earth’s suitability for life:
cohesive behavior (stickiness)
ability to moderate temperature
expansion upon freezing
versatility as a solvent
Water molecules are linked by multiple hydrogen bonds.
Cohesion - molecules staying close together because of hydrogen bonds. This is self-to-self stickiness.
Adhesion - one substance clinging to a different substance (like tape).
Capillary Action - the ability of a liquid to flow in narrow spaces without the assistance of, or even in opposition to, external forces like gravity.
Specific heat - the amount of heat that must be absorbed or lost for 1 gram of that substance to change its temperature by 1 degree Celcius.
The specific heat of water is 1 calorie/(gram x degree Celcius) this is a high specific heat. Water is very resistant to changes in temperature.
Heat of vaporization - the heat a liquid must absorb for 1 gram to be converted to a gas.
As a liquid evaporates, its remaining surface cools, a process called evaporative cooling.
Ice floats in liquid water because hydrogen bonds in ice are more ‘ordered’ making ice less dense. Water is the most dense at 4 degrees Celcius.
Organic Chemistry
All of life is built on carbon. We are ~72% water. We are ~25% carbon compounds:
Carbohydrates
Lipids
Proteins
Nucleic Acids (not covered until Genetics)
Organic chemistry: the study of carbon compounds (carbon chemistry).
Carbon atoms are versatile building blocks. They can make 4 stable covalent bonds.
 Hydrocarbons: organic molecules consisting of only carbon and hydrogen.
Hydrocarbons: organic molecules consisting of only carbon and hydrogen.
Many organic molecules, such as fats, have hydrocarbon components
Hydrocarbons can undergo reactions that release a large amount of energy
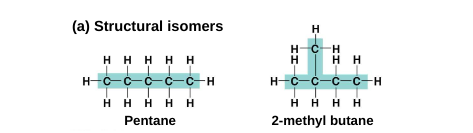
Isomers: compounds that have the same number of atoms of the same elements but different structures and properties
Structural isomers: differ in the covalent arrangement of their atoms
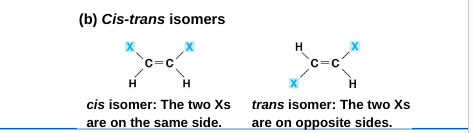
 2. Cis-trans isomers: carbons have covalent bonds to the same atoms, but the atoms differ in their spatial arrangement
2. Cis-trans isomers: carbons have covalent bonds to the same atoms, but the atoms differ in their spatial arrangement
This is due to the inflexibility of double bonds
the subtle differences in shape between such isomers can greatly affect the activities of organic molecules
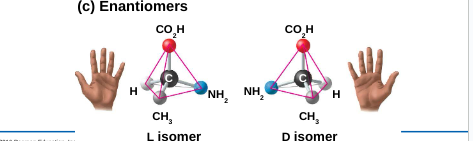
 3. Enantiomers: isomers that are mirror images of one another
3. Enantiomers: isomers that are mirror images of one another
They differ in shape due to the presence of an asymmetric carbon
Enantiomers are left-handed and right-handed versions of the same molecule
Usually, only one isomer is biologically active
 Functional groups: chemical groups that affect molecular function by being directly involved in chemical reactions
Functional groups: chemical groups that affect molecular function by being directly involved in chemical reactions
Polymer: a long molecule consisting of many similar building blocks.
These small building-block molecules are called monomers.
Dehydration reaction (synthesis): when two monomers bond together through the loss of a water molecule
Polymers are disassembled into monomers by hydrolysis, a reaction that is essentially the reverse of the dehydration reaction.
Carbohydrates
Carbohydrates: include sugars and the polymers of sugars
the simplest carbohydrates are monosaccharides or simple sugars
Uses of Carbohydrates
Fuel source in the cell
Structural (cell walls in plants)
Cell communication (blood types)
Monosaccharides: have molecular formulas that are usually multiples of CH2O
Glucose is the most common (C6 H12 O6)
Monosaccharides = monomers of carbs
A disaccharide is formed when a dehydration reaction joins two monosaccharides.
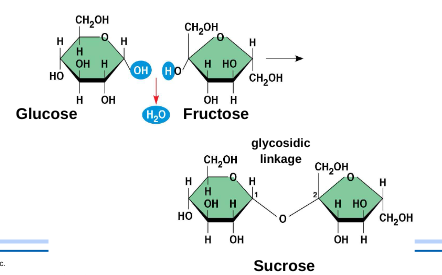 Polysaccharides: the polymers of sugars have storage and structural roles (stop being sugar)
Polysaccharides: the polymers of sugars have storage and structural roles (stop being sugar)
Starch: a storage polysaccharide of plants, consists entirely of glucose monomers. (store energy in things like potatoes so they can live underground and sprout)
Glycogen: a storage polysaccharide in animals (stored in muscles or livers)
The polysaccharide cellulose is a major component of the tough wall of plant cells - structure and support
Cellulose is a polymer of glucose but the glycosidic linkages in cellulose differ
A rope of cellulose is fiber - we can’t digest it so we use it to improve gut health
Chitin: another structural polysaccharide, is found in the exoskeleton of arthropods (crabs, insects eg.)
Chitin also provides structural support for the cell walls of many fungi
Lipids
Lipids do not form true polymers (not made from uniform monomers).
The unifying feature of lipids is having little or no affinity for water
Lipids are hydrophobic because they consist mostly of hydrocarbons, which form nonpolar covalent bonds.
Examples of important lipids are fats, phospholipids, and steroids.
Used for energy storage and part of the cell membrane. They are also hormones
Fats: constructed from two types of smaller molecules: glycerol and fatty acids
Glycerol: three-carbon alcohol with a hydroxyl group attached to each carbon
Fatty acid: consists of a carboxyl group attached to a long carbon skeleton
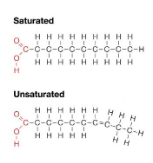
Saturated fatty acids: have the maximum number of hydrogen atoms possible and no double bonds (solid at room temperature)
Unsaturated fatty acids: have one or more double bonds (liquid at room temperature)
 The major function of fats is energy storage
The major function of fats is energy storage
In a phospholipid, two fatty acids and a phosphate group are attached to glycerol
the two fatty acid tails are hydrophobic, but the phosphate group and its attachments form a hydrophilic head
Phospholipids are major constituents of cell membranes
Steroids: lipids characterized by a carbon skeleton consisting of four fused rings
Cholesterol: is an important steroid and is a component in animal cell membranes (adds flexible rigidity to cell walls) (type of steroid)
Proteins
Most structurally and functionally diverse group
Function: involved in almost everything
enzymes (pepsin, DNA polymerase)
structure (keratine, collagen)
carriers and transport (hemoglobin, aquaporin)
cell communication
signals (insulin and other hormones)
receptors
defense (antibodies)
movement/motor (actin and myosin)
storage (bean seed proteins)
Structure
monomer = amino acids
20 kinds of different amino acids
polymer = polypeptide
protein can be one or more polypeptide chains folded and bonded together
large and complex molecules
complex 3-D shape
Amino Acids
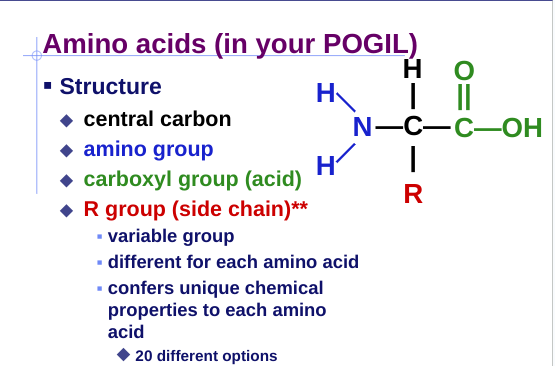
Peptide bonds:
covalent bond between NH2 (amine) of one amino acid & COOH (carboxyl) of another (dehydration synthesis)
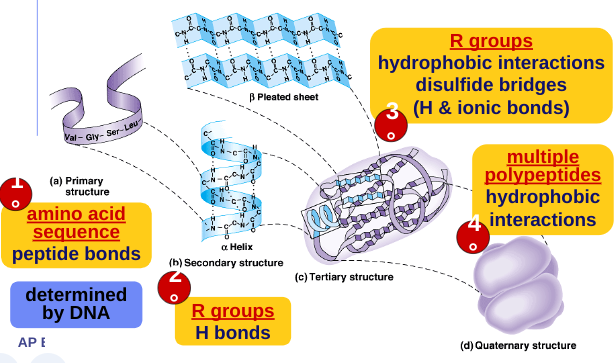
Primary Structure:
amino acids in a chain
amino acid sequence determined by gene (DNA)
 Secondary structure:
Secondary structure:‘Local folding’
folding along short sections of polypeptide
interactions between adjacent amino acids
H bonds
weak bonds between R groups
forms sections of the 3-D structure
α - helix
β - pleated sheet
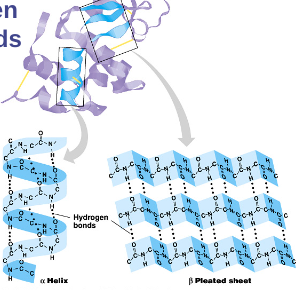 Tertiary structure:
Tertiary structure:
“Whole molecule folding”
interactions between distant amino acids
hydrophobic interactions
cytoplasm is water-based
nonpolar amino acids cluster away from water
H bonds and ionic bonds
disulfide bridges
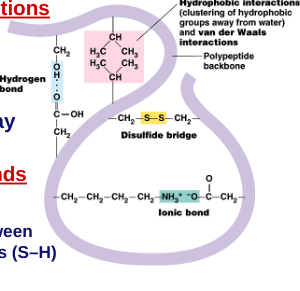 Quaternary structure:
Quaternary structure:
more than one polypeptide chain bonded together
only then does polypeptide become a functional protein
hydrophobic interactions
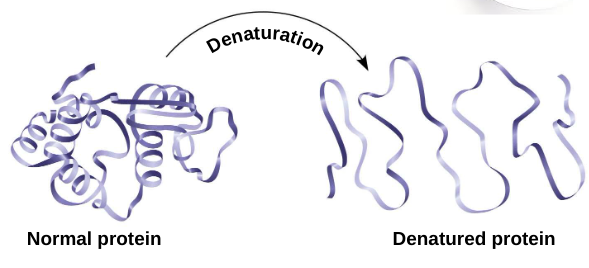
 Protein denaturation:
Protein denaturation:
the unfolding or destruction of a protein
conditions that disrupt H bonds, ionic bonds, disulfide bridges
caused by:
temperature
pH
salinity
alters secondary and tertiary structure
destroys functionality
some proteins can return to their functional shape after denaturation, many cannot
 Nucleic Acids
Nucleic Acids
There are two types of nucleic acids:
Deoxyribonucleic acid (DNA)
Ribonucleic acid (RNA)
Monomer: nucleotide (sugar, phosphate, nitrogenous base)