Ch.4 Energy Enzymes lecture notes/flashcards-Honey Ortiz
Chapter 4: Ground Rules of Metabolism
Key Concepts
Energy
Metabolism
Enzymes
Energy in Ecosystems
Most life forms derive energy from the sun.
Photosynthesis: Plants convert sunlight into energy.
Energy Transfer:
Herbivores consume plants for energy.
Carnivores eat herbivores.
Decomposers break down waste, recycling nutrients into the ecosystem.
Understanding Energy
Definition: Energy is the capacity to do work.
Types of Energy:
Kinetic Energy: Energy associated with motion.
Potential Energy: Stored energy found in chemical bonds.
Energy Transformations
Thermodynamics: Study of energy transfer involving physical matter.
1st Law of Thermodynamics: Energy cannot be created or destroyed, only transformed or transferred.
Energy has a one-way flow; often lost as heat.
2nd Law of Thermodynamics: Energy disperses spontaneously, spreading out over time.
Energy from the Sun
The sun is the primary energy source for most life on Earth.
Energy harvested undergoes multiple transfers before becoming permanently dispersed.
Chemical Bonds: Resists energy's spontaneous dispersal, acting as potential energy stores.
Metabolism
Definition: Refers to all chemical reactions in living organisms.
Metabolic Pathways: Series of reactions that build, rearrange, or break down organic molecules, controlled by specific enzymes.
Pathways are well-controlled and serve specific biological purposes.
Types of Metabolic Pathways
Catabolic Pathways: Generate energy by breaking down larger molecules.
- cat knocks down the tower of blocksAnabolic Pathways: Require energy to build up larger molecules.
- Ana builds a tower with blocksImportance: Both pathways are essential for maintaining cellular energy balance.
Reactants and Products
Reactant: Molecule that enters a reaction and undergoes change.
Product: Molecule produced as a result of the reaction.

Enzymatic Action
Catalysts: Agents that speed up chemical reactions without being used up/consumed.
Enzymes: Proteins that act as catalysts in living cells.
Role: Metabolism heavily relies on enzymes.
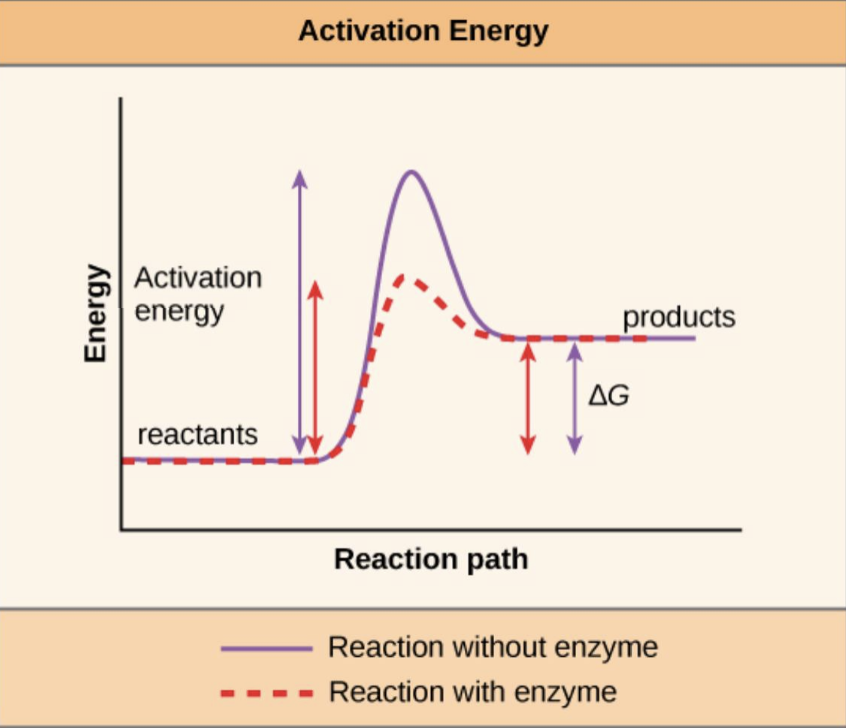
Activation Energy
Definition: The initial energy input required to start a reaction.
Methods to Overcome activation energy:
High heat (form of energy).
Utilizing enzymes to lower activation energy.
Enzymes facilitate reactions by applying strain to bonds, aiding transition to product formation.
Properties of Enzymes
Enzymes often end in “ase”
Specificity: Each enzyme binds to specific substrates (reactants), undergoing particular changes (e.g., lactase (enzyme) only breaks down lactose (substrate).
Models:
Lock and Key Model: Specificity of enzyme to substrate.
Induced Fit: Enzyme changes shape when substrate binds, allowing reaction to proceed.
Enzymes remain free to catalyze further reactions once products leave the active site.
Product: outcome of a reaction
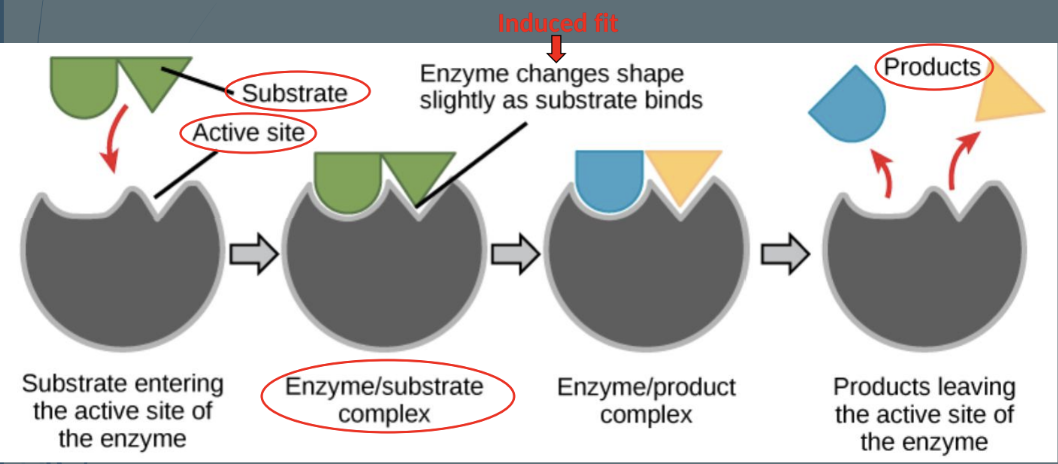
Enzyme Terminology
Substrate: The reactant that an enzyme acts on.
Active Site: The region on the enzyme where substrates bind and reactions occur.
Induced Fit: Minor changes in enzyme shape when substrate binds.
Enzyme-Substrate Complex: Temporary molecule formed when an enzyme binds its substrate.
Product Formation: New molecules formed as the outcome of the reaction.
Environmental Effects on Enzymes
Environmental factors (pH, temperature, salt concentration) influence enzyme shape and job/function.
Enzymes typically function optimally within a narrow range of conditions (temp and pH).
Enzymes and pH
Example of two digestive enzymes:
Pepsin: Functions at pH 2 in the stomach.
Trypsin: Functions at pH 7.5 in the small intestine.
Enzyme Inhibition
Competitive Inhibition: Inhibitor binds to the active site; competes with the substrate.
Noncompetitive Inhibition: Inhibitor binds to allosteric site, altering active site functionality.
Summary
Enzymes are crucial for metabolism, affected by their environment and capable of being inhibited through various mechanisms.