2.1.5 Transport across membranes
2.1.5 Biological membranes (d and some of e)
Transport across membranes - Notes
2.1.5d: (i) the movement of molecules across membranes (ii) practical investigations into the factors affecting diffusion rates in model cells
- Define the terms “simple diffusion”, “facilitated diffusion”, “osmosis”, “passive transport”, “active transport”, “endocytosis”, “exocytosis”, “pinocytosis” and “phagocytosis”. (F)
- Explain the difference between a channel protein and a carrier protein and link this to their functions.
- State 3 particular examples of channel proteins and 3 carrier proteins and for each describe the role that they perform. (S+C)
- Explain the importance of the structure of ATP in active transport.
- List the factors affecting the rate of diffusion of a molecule. (F)
- Explain, in a paragraph and with a diagram, how substances can move across a membrane by simple diffusion.
- State which types of molecule can move across a membrane by simple diffusion. (F)
- Explain, in a paragraph and with a diagram, how substances can move across a membrane by facilitated diffusion (include protein specificity). (F)
- Explain, in a paragraph and with a diagram, how substances can move across a membrane by active transport (include protein specificity). (F)
- Explain, in a paragraph and with a diagram, how substances can move into a cell by endocytosis (using phagocytosis as an example). (F)
- Explain, in a paragraph and with a diagram, how substances can move out of a cell by exocytosis (using secretion as an example). (F)
- Identify which transport mechanisms require an input of energy from ATP. (F)
- Explain why it is easier for an oxygen molecule to diffuse across a membrane than a water molecule.
- Explain why steroid hormones can easily cross a membrane by simple diffusion.
- Explain why ions are only able to cross a membrane through a protein channel.
- Describe how to carry out an investigation to investigate how the rate of diffusion is affected by surface area.
- Describe and explain the results you would expect to see in an investigation into how the rate of diffusion is affected by surface area.
- Describe how to carry out an experiment to investigate how the rate of diffusion is affected by temperature.
- Describe and explain the results you would expect to see in an investigation into how the rate of diffusion is affected by temperature.
2.1.5e: (i) the movement of water across membranes by osmosis and the effects that solutions of different water potential can have on plant and animal cells (ii) practical investigations into the effects of solutions of different water potential on plant and animal cells.
- Define the terms “solvent”, “solute” and “solution”. (F)
- Define the term “water potential”, state the symbol for water potential, state the water potential of pure water and explain why water potential cannot have a positive value. (F)
- Define the terms “water potential gradient” and “net movement of water”. (F)
- Draw a table to compare the effects of external solutions, with different water potentials in comparison to the water potential in a cell, on animal and plant cells (include a description of relative water potentials, osmosis and the overall effect on the cell). (F)
- Define the terms “plasmolysis”, “protoplast”, “crenation”, “turgid”, “cytolysis” and “haemolysis”.
- Describe how to carry out an experiment to investigate the effects of solutions of different water potential on plant and animal cells.
- Describe and explain the results you would expect to see from an experiment to investigate the effects of solutions of different water potential on plant and animal cells.
- Describe the meaning of the symbols: =, <, <<, >>, >, ∝, ~ [covered in a different document]
- Explain why plant cells don’t burst by osmosis by referring to solute potential and pressure potential.
- Write an equation to link the water potential of a cell with its pressure potential and its solute potential.
- Explain what is meant by “uncertainty in measurements” and define the terms “absolute uncertainty” and “relative uncertainty or percentage error”. [covered in a different document]
- Explain how to calculate the percentage error of a measurement (and when data are combined e.g. to obtain data on the change of a particular value over time). [covered in a different document]
- Describe how to plot two variables from data into an appropriate graphical form. [covered in a different document]
- State the equation for a straight line. [covered in a different document]
- State the meaning of the terms “m” and “c” in the equation for a straight line and describe how to find m and c from a graph. [covered in a different document]
- Describe how to find where a straight line intercepts the x-axis both from a graph and from the equation for the straight line. [covered in a different document]
- Explain the significance of where a line of best fit crosses the x-axis in a graph showing how external concentration affects mass change in plant tissues.
- Describe how to evaluate an experimental design (including limitations of the experiment). [covered in a different document]
- Describe how to identify possible improvements to an experimental design. [covered in a different document]
- Define the term “anomaly” and explain how to identify anomalies in experimental measurements (also describe how anomalies can be dealt with) [covered in a different document]
- Describe the considerations that need to be taken into account when drawing conclusions from data. [covered in a different document]
- Define the terms “precision”, “accuracy”, “repeatability”, “reproducibility”, “resolution” and “validity” in relation to experimental design and scientific equipment. [covered in a different document]
Diffusion
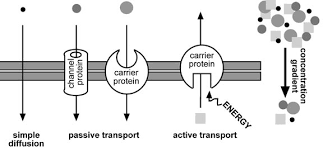 Diffusion is the net movement of molecules or ions from an area of high concentration to an area of low concentration. Diffusion always occurs down a concentration gradient. Diffusion does not require chemical energy so it is a passive transport process. In ‘simple’ diffusion small (e.g. water, oxygen, carbon dioxide) or fat soluble (non-polar) molecules (e.g. steroid hormones, fat soluble vitamins, alcohol) diffuse straight through the phospholipid part of the membrane. This is because they can dissolve in the fatty layer made of the hydrophobic tails of the phospholipids.
Diffusion is the net movement of molecules or ions from an area of high concentration to an area of low concentration. Diffusion always occurs down a concentration gradient. Diffusion does not require chemical energy so it is a passive transport process. In ‘simple’ diffusion small (e.g. water, oxygen, carbon dioxide) or fat soluble (non-polar) molecules (e.g. steroid hormones, fat soluble vitamins, alcohol) diffuse straight through the phospholipid part of the membrane. This is because they can dissolve in the fatty layer made of the hydrophobic tails of the phospholipids.
Although water is polar it is so small that it can, to a certain extent, diffuse across the phospholipid bilayer. However, the majority of cells also have channel proteins called aquaporins that allow water to diffuse through.

Osmosis
Osmosis is a special case of diffusion just involving water. Osmosis is the net movement of water, by diffusion, through a partially permeable membrane from a solution of higher water potential to a solution of lower water potential, down a water potential gradient.
Facilitated diffusion
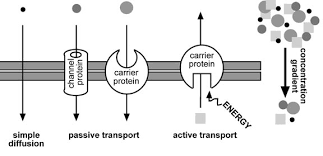 Facilitated diffusion is also a special case of diffusion. In this case ions (e.g. sodium or calcium ions) or polar molecules (e.g. glucose) diffuse through membranes with the help of channel proteins or carrier proteins. The carrier proteins have binding sites that are specific to one type of molecule. When a molecule binds to the carrier, the carrier changes shape thereby moving the molecule across the membrane. Channel proteins are either permanently open or have gates that can open (and so allow diffusion) or close (not allowing diffusion). The channels within channel proteins are specific for particular molecules. Facilitated diffusion does not require chemical energy.
Facilitated diffusion is also a special case of diffusion. In this case ions (e.g. sodium or calcium ions) or polar molecules (e.g. glucose) diffuse through membranes with the help of channel proteins or carrier proteins. The carrier proteins have binding sites that are specific to one type of molecule. When a molecule binds to the carrier, the carrier changes shape thereby moving the molecule across the membrane. Channel proteins are either permanently open or have gates that can open (and so allow diffusion) or close (not allowing diffusion). The channels within channel proteins are specific for particular molecules. Facilitated diffusion does not require chemical energy.
Active transport
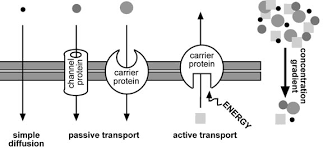 Active transport is the movement of substances from where they are less concentrated to where they are more concentrated. This is against the concentration gradient. Like facilitated diffusion this process requires carrier proteins which change shape when the molecule binds to them. However in this case the shape change requires chemical energy in the form of ATP. There is an ATP binding site (that is a complementary shape to ATP) and the hydrolysis of ATP into ADP + Pi releases the energy needed to change the shape of the carrier protein. Active transport is used to reabsorb glucose in the kidneys and for plants to absorb ions (e.g. nitrate and phosphate ions) from the soil.
Active transport is the movement of substances from where they are less concentrated to where they are more concentrated. This is against the concentration gradient. Like facilitated diffusion this process requires carrier proteins which change shape when the molecule binds to them. However in this case the shape change requires chemical energy in the form of ATP. There is an ATP binding site (that is a complementary shape to ATP) and the hydrolysis of ATP into ADP + Pi releases the energy needed to change the shape of the carrier protein. Active transport is used to reabsorb glucose in the kidneys and for plants to absorb ions (e.g. nitrate and phosphate ions) from the soil.
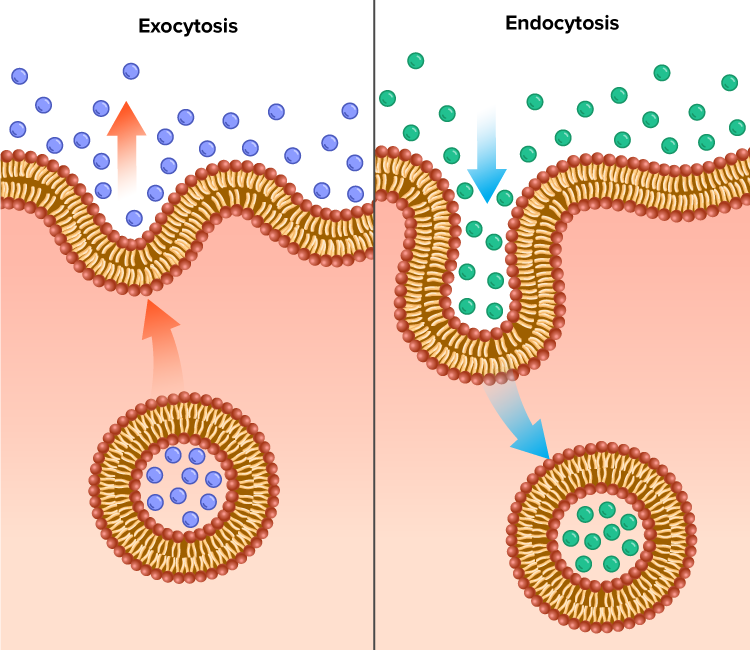 Endo and Exocytosis
Endo and Exocytosis
Exocytosis involves the use of vesicles, created by the Golgi apparatus, to transport materials (e.g. mucus secretion) out of the cell. Vesicles bind with the cell-surface membrane and, because the membrane is fluid, it parts and lets the contents of the vesicle out. Endocytosis is the reverse process where materials are brought into the cell by the formation of vesicles. Both processes require chemical energy in the form of ATP to move the vesicles along microtubule tracks. Pinocytosis is the absorption of liquids by endocytosis and phagocytosis is the absorption of solids by endocytosis.
Factors affecting rate of diffusion
The rate of diffusion is affected by temperature. As temperature increases so does the rate of diffusion. It is also affected by the concentration gradient. The steeper the gradient the faster the rate of diffusion.
If we consider diffusion in an organism the rate of diffusion could be in relation to how long it takes for enough of a molecule to get somewhere or how long it takes for enough of a molecule to enter an organism.
In these cases, as well as being affected by temperature and concentration gradient, the rate of diffusion is affected by the distance diffusion needs to cover and by the surface area over which diffusion occurs.
Where facilitated diffusion is occurring the rate of diffusion is also affected by the number of channels available for diffusion.
Fick’s law summarises some of the factors that affect the rate of diffusion. It states that:
We will look at this a lot more when we study exchange surfaces.
Experiments into factors affecting the rate of diffusion
The rate of diffusion can be studied using Agar blocks containing phenolphthalein in acid. The blocks are pink initially and they turn colourless from the outside inwards as the acid diffuses into them.
By adjusting the size and shape of the blocks you could investigate how the surface area of the block affects the volume of the block that turns colourless in a certain amount of time, or what percentage of the block turns colourless for blocks of different surface area to volume ratio.
By keeping the size and shape of the block constant you could investigate the effect of temperature or concentration gradient on the rate of diffusion through the block.
A focus on osmosis
Osmosis is all about the movement of water from one solution on one side of a partially permeable membrane to the solution on the other side. A solution is a liquid in which the solute is uniformly distributed within the solvent. A solute is a chemical that has dissolved in the solvent to form the solution and the solvent is the liquid in which the solute has been dissolved. In this context the solvent is water and the solutes are polar or charged ions or molecules.
Different solutions have different concentrations of solutes in them and therefore a different concentration of ‘free’ water molecules. If two solutions with different concentrations of ‘free’ water molecules are separated by a partially permeable membrane, that the solute cannot cross, water will diffuse from the one with the higher concentration of ‘free’ water to the one with the lower concentration of ‘free’ water.
Note that diffusion involves the net movement of water. This is the overall movement of water from one area to another (taking into account that some water molecules will be moving from A to B whereas others will be moving from B to A).
Because the term ‘concentration’ refers to the solute rather than the solvent we need a different term to describe the concentration of ‘free’ water. The term used is ‘water potential’. Water potential quantifies the tendency of water to move from one area to another. It is the potential energy the solution has with reference to pure water. A water potential gradient is the difference in water potential between two areas.
The symbol for water potential is Ψ (called ‘Psi’) and it is a pressure and so is measured in Pascals (Pa). The water potential of pure water is 0kPa. Because pure water has a water potential of 0kPa and every solution has a lower concentration of ‘free’ water than that, the water potential of all solutions are negative values. 0kPa is the highest water potential.
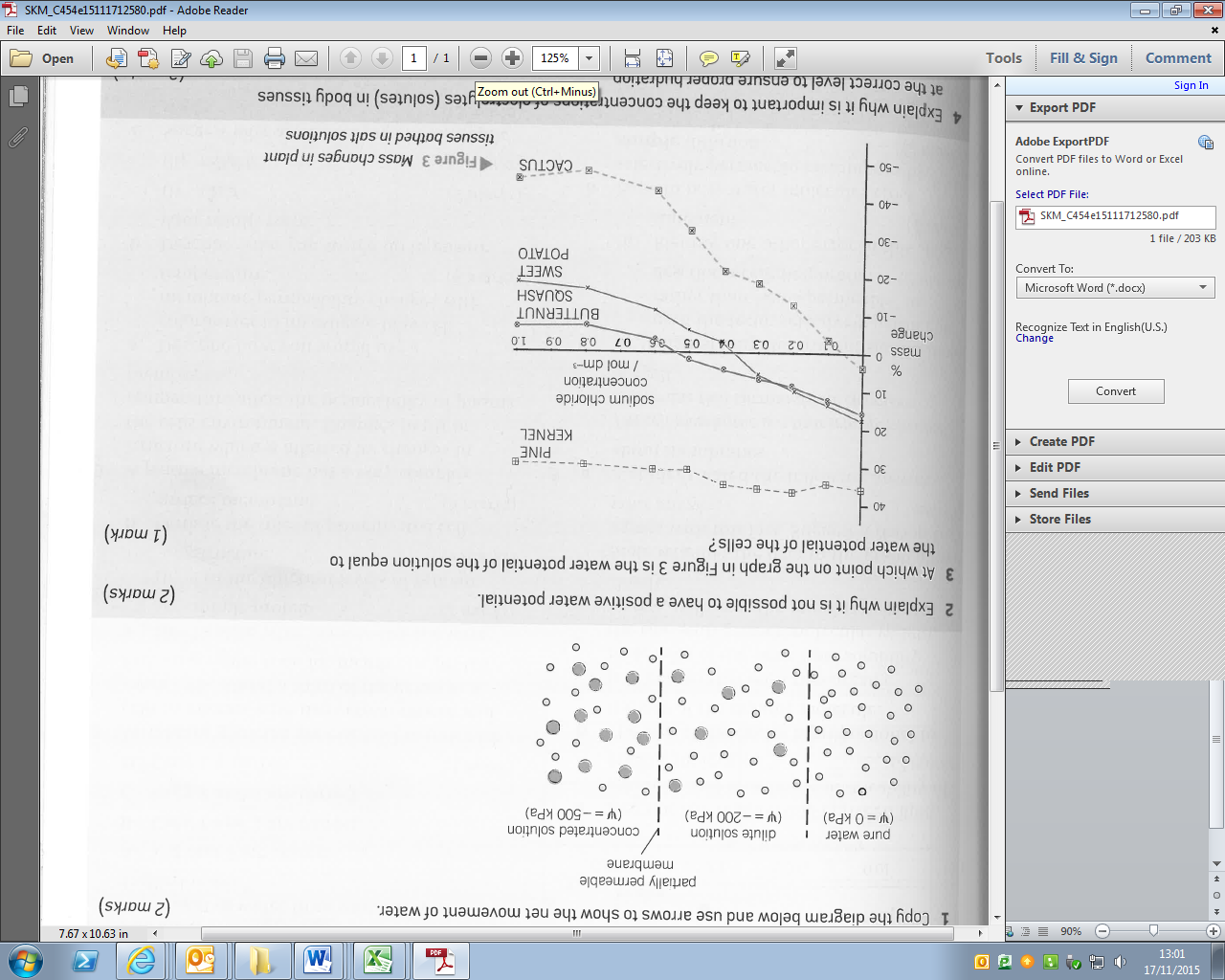
Draw arrows on the diagram to show the net flow of water between each solution.
The terms hypotonic, isotonic and hypertonic can be used to compare solutions. Two solutions of the same water potential are called isotonic solutions. If two solutions have different water potentials the one with the higher water potential is called hypotonic and the one with the lower water potential is called hypertonic. The term ’tonic’ refers to the solute and ‘hypo’ means ‘below’ and ‘hyper’ means ‘above’.
If cells are placed in solutions that are not isotonic with their cytoplasm then there will be a net flow of water either into or out of the cell and this will have consequences for the cell. The table below summarises what happens to animal and plant cells when placed in solutions of different water potentials.
Animal cells | |||
Water potential of external solution compared to inside the cell | Higher (less negative) Hypotonic | Equal Isotonic | Lower (more negative) Hypertonic |
Net movement of water | Into cell | No net movement | Out of cell |
State of cell | Swell and burst - cytolysis | Normal | Crenated |
Plant cells | |||
Water potential of external solution compared to inside the cell | Higher (less negative) Hypotonic | Equal Isotonic | Lower (more negative) Hypertonic |
Net movement of water | Into cell | No net movement | Out of cell |
State of cell | Turgid | Normal (you’d call this turgid) | Plasmolysed |
Plasmolysis: the contraction of the protoplast in a plant cell due to loss of water from the cell. It is observed by the protoplast being seen to have pulled away inwardly from the cell wall.
Protoplast: a plant cell without its cell wall (or all the parts of a plant cell except the cell wall)
Crenation: animal cells acquiring a notched or scalloped appearance due to shrinkage caused by loss of water
Turgid: a plant cell is turgid when its protoplast is pushing against the cell wall.
Cytolysis: the bursting of cells.
Haemolysis: the bursting of red blood cells.
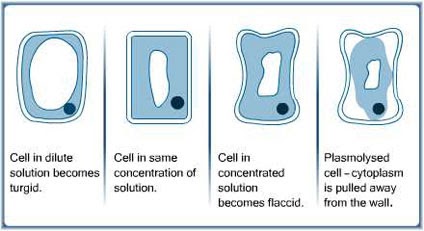
What’s in the spaces between the protoplast and the cell wall in the plasmolysed cell?
The most notable difference between plant and animal cells in relation to osmosis is that animal cells will burst (undergo lysis) if placed in a hypotonic solution whereas plant cells will become turgid and not burst.
When a plant cell takes in water by osmosis it enters the vacuole and the vacuole swells. This pushes the cytoplasm and plasma membrane up against the cell wall. The cell wall is inelastic and so pushes back on the protoplast preventing it getting any bigger, preventing any more water entering and so preventing bursting.
Solution potential and pressure potential
The cytoplasm of a plant cell contains dissolved solutes which give it a solute potential, ψS. Solute potential is always negative.
The wall of the cell exerts a pressure on the cell contents giving a pressure potential, ψP.
The pressure potential opposes water entering the cell.
Because a larger pressure potential increases the tendency of a solution to lose water it is given positive values.
The overall ψ of the cell is calculated using the equation: Ψ = ψS + ψP

Investigating osmosis
You can investigate osmosis in different plant or animal tissues by putting them in solutions of different water potential and then seeing what happens.
You can see what happens by looking at cells under a microscope or by seeing how much a tissue sample changes in mass.
The graph below shows the percentage change in mass when plant samples from different species are placed in solutions of different water potential.
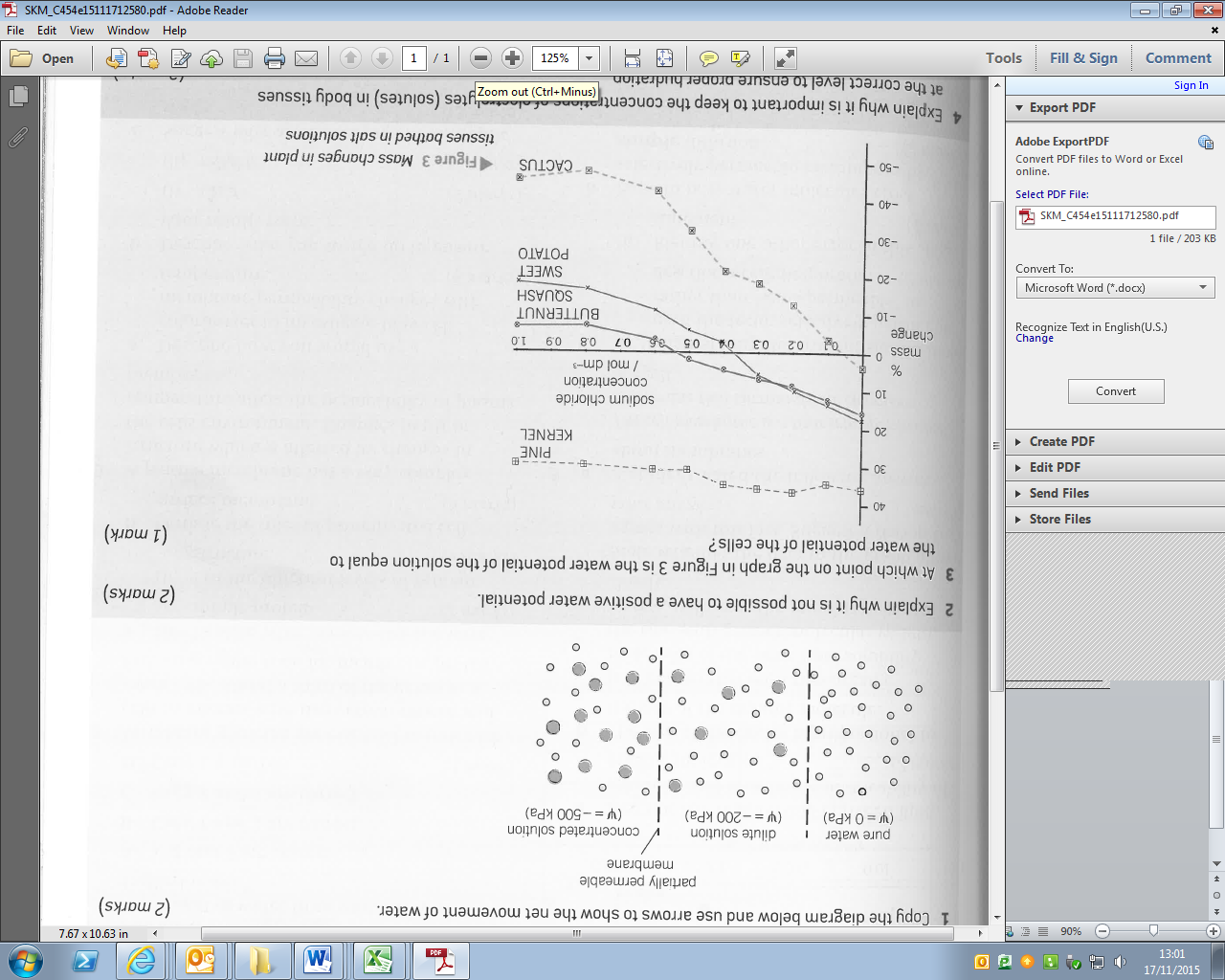
What is the significance of the point at which each line crosses the x-axis?
Answer:
The water potential scale summary
Fewer water molecules
Pure water = 0kPa water potential
If a cell is placed in a hypotonic solution:
water would diffuse into the cell by osmosis.
Plant cells would become turgid, animal cells would swell and burst (this is cytolysis)
If a cell is placed in an isotonic solution:
there would be no net flow of water
If a cell is placed in a hypertonic solution:
water would diffuse out of the cell by osmosis.
Plant cells would become plasmolysed, animal cells would become crenated
Concentrated solution = very low water potential
Many water molecules
No solutes
higher water potential
Many solutes
lower water potential
(more negative)