Key topics (APES)
Review straight from the slides
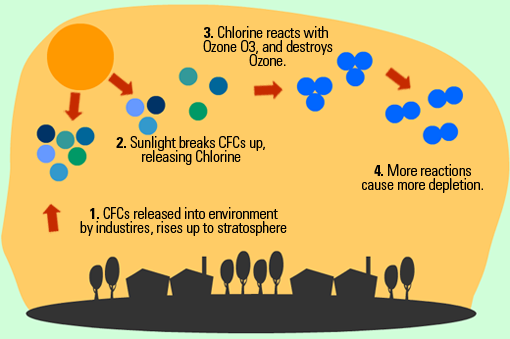
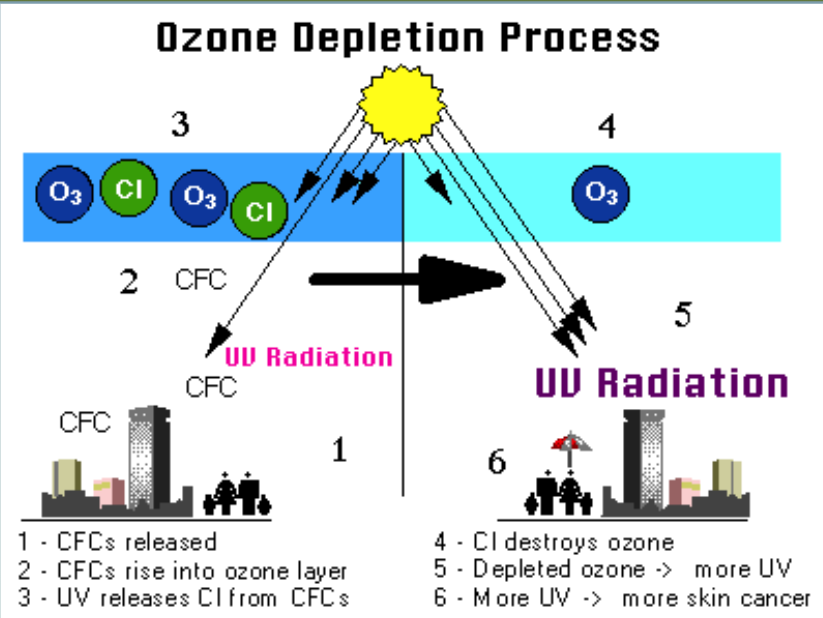
Ozone Depletion
CFCs are man-made substances found in coolants and aerosols. In the stratosphere under UV light one of the three Cl- molecules can come off, which reacts with Ozone (O3) to create O2 + ClO. This depletes the ozone layer. Other free O- molecules interact with the ClO to create O2 and Cl-, which causes the cycle to repeat itself.
Tropospheric Ozone Depletion
In the troposphere, NO2 from cars reacts in sunlight to form NO + O-.
The lone oxygen will react with O2 to form Ozone (O3) in the troposphere, which is BAD.
Ozone is: “Good up high (in the stratosphere), bad nearby (troposphere)
This NO will go on to react with VOC (Volatile Organic Compounds) to form Photochemical Oxidants.
Photochemical Oxidants combined with Ozone will create Photochemical smog.
Importance of Wetlands
Threatened by drainage and development
Ecosystem services provided include
Maintain drinking quality
Flood control
Water filtration
Commercial fisheries
Recreation
Wildlife habitat (contributes to food or ecotourism)
The Nitrogen Cycle
 Nitrogen Fixation
Nitrogen Fixation
The process where certain bacteria convert nitrogen gas from the air into a form plants can use.
Example: Rhizobium bacteria in legume roots help plants get nitrogen.
Importance: Provides essential nitrogen for plant growth.
N₂ + 3H₂ → 2NH₃
Ammonification
The process where organic nitrogen from dead organisms is converted into ammonia by decomposers like bacteria. Plants can then use this ammonia to make proteins.
Example: Bacteria break down organic nitrogen compounds in dead organisms into ammonia. For instance, in soil, bacteria decompose dead plants and animals, releasing ammonia as a byproduct.
Importance: It converts organic nitrogen from dead organisms into ammonia, which plants can use for growth.
organic nitrogen compounds → NH3
Nitrification
The process where bacteria convert ammonia into nitrites, then into nitrates.
Example: Ammonia is converted to nitrite by Nitrosomonas bacteria, then to nitrate by Nitrobacter bacteria in soil. For instance, in agricultural fields, ammonia from fertilizers is transformed into nitrate, which plants can absorb for growth.
Importance: Creates essential nutrients for plants. This process makes nitrogen available for plant uptake, promoting growth and productivity in ecosystems.
NH3 → NO3-
Assimilation
The process where plants and animals take up nitrogen compounds like nitrates from the soil to build proteins and other essential molecules for growth and development.
Example: Plants absorb nitrate or ammonium ions from the soil and incorporate them into their proteins, DNA, and other organic molecules.
Importance: It converts inorganic nitrogen into organic forms like proteins, DNA, and chlorophyll, making nitrogen available for living organisms.
Incorporation of NH3 and NO3- into biological tissues
or
NH4+ + 2O2 -> NO3- + H2O + 2H+
Dentrification
The process where bacteria convert nitrates in the soil back into nitrogen gas, releasing it into the atmosphere.
Example: Bacteria convert nitrates in soil back into nitrogen gas, releasing it into the atmosphere.
Importance: Converts nitrates back into nitrogen gas, completing the nitrogen cycle and replenishing the atmosphere with nitrogen.
NO3 → N2
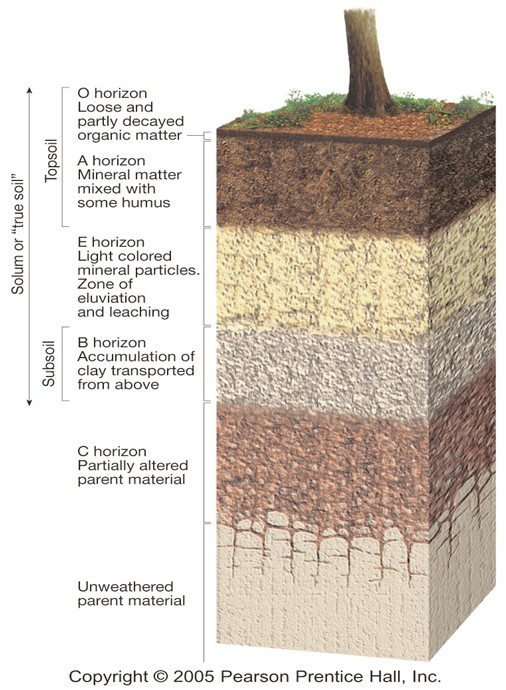
Soil Components
O Horizon
Organic matter in various stages of decomposition
A Horizon (topsoil)
Zone of overlying organic material mixed with underlying mineral material
E Horizon
Zone of leaching of metals and nutrients; occurs in some soils beneath either the O horizon or the A horizon
B Horizon (subsoil)
Zone of accumulation of metals and nutrients
C Horizon (subsoil)
Least-weathered portion of the soil profile, similar to the parent material
 Vocab
Vocab
Horizon: Each layer of soil
Soil profile: The cross-section of soil as a whole
(up to six major horizons may occur in a soil profile)
Topsoil: inorganic and organic material most nutritious for plants
Leaching: Dissolved particles move down through horizons
Climate Change
Some greenhouse gasses are produced by human activity:
Burning of fossil fuels
Agricultural practices
Deforestation
Landfills
Industrial production
Carbon Dioxide
Although carbon dioxide is not the most potent greenhouse gas, it is extremely abundant
It is a major contributor to global warming
Human activities have boosted atmospheric concentrations from 280 ppm to 383 ppm
At their highest levels in more than 650,000 years
Impacts of the Increase in Greenhouse Gases
Melting of polar ice caps (in Greenland and Antarctica)
Melting of many glaciers around the world
Melting of permafrost
Rising of sea levels due to the melting of glaciers and ice sheets and as the water warms it expands
Heat waves
Cold spells
Change in precipitation patterns
Increase in storm intensity
Shift in ocean currents
Ocean Acidification
Coral reefs can be bleached due to the increase in water temperature
This affects coral symbiotes and makes them more susceptible to diseases
Ocean Acidification: The other CO2 problem
Surface waters have increased in acidity by 30% since 1800
Could reach dangerous levels before 2050
CO2 combines with water to form carbonic acid (H2CO3)
Threatens corals, snails, and other organisms with shells
Organisms
Organisms are adapted to their environments, so they are affected when those environments change
Global warming modifies temperature-dependent phenomena
Timing of migration and breeding change
Spatial shifts in the range of organisms
Animals and plants will move toward the poles or upward in elevation
20-30% of all species will be threatened with extinction
Plants act as carbon sinks; fewer plants means more CO2 in the atmosphere
Solutions
Mitigation: Pursue actions that reduce greenhouse gas emissions, in order to lessen the severity of future climate change
Renewable energy sources, farm practices to protect soil integrity preventing deforestation
Adaptation: Accept climate change is happening and pursue strategies to minimize its impacts on us
Criticized as sidestepping
Both are necessary
Carbon Offset: A voluntary payment to another entity intended to enable that entity to reduce the greenhouse emissions that one is unable or unwilling to reduce oneself
Becoming popular among utilities, businesses, universities, governments, and individuals trying to achieve carbon neutrality, where no net carbon is emitted
Carbon offsets fall short
A lack of oversight to make sure that the offset money accomplishes what it is intended for
Chem Review
pH
pH is a measure of the amount of H+ and OH- ions in a solution
Acids have a higher concentration of H+ and Bases have a higher concentration of OH-
The pH scale is logarithmic, so each value is 10x greater than the next higher value
Formation of Acids in the Environment
NOx (from cars) + H2O → HNO3 (Nitric acid)
SOx (from coal) + H2O → H2SO4 (Sulfuric acid)
CO2 (from fossil fuels) + H2O → H2CO3 (Carbonic acid)
The formation of an acid causes the pH to decline
Environments that become acidic from acid rain or acid mine drainage may be remedied by adding a base such as limestone. Acid rain results in the loss of nutrients from soil (clay is attracted to the acid and releases metals) and metals such as AL3+ may be leached out of the soil and runoff into groundwater. Acid rain leads to general forest decline.
The ocean becomes acidic due to carbonic acid from excess release of CO2 from burning fossil fuels. Ceasing the use of fossil fuels will help prevent the problem, but there is remediation - you can not add enough limestone to raise the pH of the ocean.
Dissolved Oxygen
Gases dissolve better in cooler-temperature liquids. As temperature increases, less gas dissolves. As temperatures decrease, more gas dissolves. As temperatures warm or cool due to climate change, more or less oxygen can be dissolved in the waters. Cooler temperature waters hold more dissolved oxygen (DO) while warmer waters hold less. This can impact an organism’s range of tolerance. As our oceans warm, they absorb and store less CO2 and less DO. If sediment erodes and runs off into a waterway, the dark soil absorbs sunlight, warming the waters and causing DO to decline.
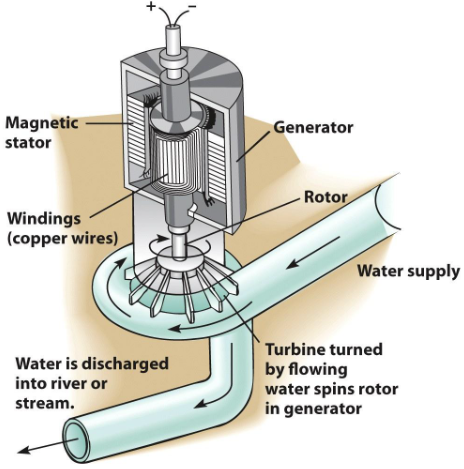
Electricity
The flow of electrons in a wire
Can be generated from almost any energy source
Energy sources spin a turbine
The turbine turns a generator
A bundle of wires spins around a magnet or vice versa
Air Pollution
Tropospheric Ozone and Photochemical Smog Formation
Tropospheric Ozone Formation:
Formed by reactions of nitrogen oxides and volatile organic compounds in the presence of sunlight.
Precursor pollutants are released from human activities like vehicle emissions.
Photochemical Smog Formation:
Results from the interaction of sunlight with pollutants like nitrogen oxides and volatile organic compounds.
Leads to the formation of ground-level ozone and other harmful compounds.

Solutions
Decreased use of fossil fuels
Improved efficiency
Use of electrostatic precipitators and scrubbers
Dams
Advantages | Disadvantages |
|---|---|
|
|

Much of hydropower in recent years has been from enormous dams
Human Displacement
Ecosystem Destruction
Wildlife Losses
Large-Scale Flooding Due to Dam Failures
Sedimentation
Herbicide Contamination
Evaporative Losses
Nutrient Flow Retardation
Rotting of submerged vegetation kills fish, acidifies water, produces greenhouse gases
Schistosomiasis - a human disease caused by a parasitic fluke that lives in snails, which like the slow-moving water behind dams
Eutrophication
Eutrophication Process:
Excessive Nutrients: Nutrient runoff enters water.
Algal Bloom: Nutrients promote algal growth.
Algae Die-off: Algae die, sink, and decompose.
Oxygen Depletion: Decomposition reduces oxygen.
Dead Zone Formation: Low oxygen levels harm aquatic life.
Biochemical Oxygen Demand - the amount of dissolved oxygen consumed by aquatic microorganisms.
Used as a test for organic waste contamination.
Dissolved Oxygen Content - a measure of dissolved oxygen in the water
The effects of oxygen-demanding wastes on rivers depend on river water's volume, flow, and temperature.
Oxygen Sag - Oxygen levels decline downstream from a pollution source as decomposers metabolize waste materials
Biochemical Oxygen Demand - The amount of dissolved oxygen consumed by aquatic microorganisms.
Used as a test for organic waste contamination.
Dissolved Oxygen Content - a measure of dissolved oxygen in the water
The effects of oxygen-demanding wastes on rivers depend on the volume, flow, and temperature of river water.
Oxygen Sag - Oxygen levels decline downstream from a pollution source as decomposers metabolize waste materials
During hot weather or drought
Dense growths of algae and cyanobacteria
Impacts of Eutrophication
The level of nitrates discharged from the Mississippi River into the Gulf of Mexico tripled since the 1950s
This causes severe depletion of dissolved oxygen
Food web disruption
Many species cannot migrate away from the area and die
Which causes deaths of seabird and marine mammal species that depend on dying fish and shellfish
Human Factors
Dredging and straightening increase the flow of nutrients
Removal of wetlands that act as filters for pollutants
Can only be repaired with:
Time
Small areas that can be aerated
Prevented by:
Education
Riparian zones
Plant ground cover or crops to absorb fertilizer
Sustainable agriculture practices
Landfills

Solid Waste Management Terms:
Groundwater Monitoring: Monitoring water quality to prevent contamination.
Methane Collection: Capturing methane gas from waste for energy.
Solid Cap: Covering waste to prevent water infiltration.
Open Cell: Waste disposal area without liners.
Leachate: Liquid formed by water passing through waste.
Leachate Collection: System to collect and treat leachate.
Closed Cell: Waste disposal area with liners.
HDPE Liner: High-density polyethylene liner to contain waste.
Gravel: Used for drainage in waste disposal areas.
Clay: Natural material used for sealing waste containment areas.
The Carbon Cycle
The Carbon Cycle: The process through which carbon is exchanged between the atmosphere, oceans, soil, and living organisms.
Importance: It regulates the Earth's climate by balancing carbon dioxide levels in the atmosphere, supports plant growth through photosynthesis, and is essential for the survival of all living organisms.
Processes of the Carbon Cycle
1. Photosynthesis
Plants absorb CO2 from the atmosphere
Convert CO2 into glucose through photosynthesis
2. Respiration
Organisms release CO2 back into the atmosphere
Breakdown of glucose for energy
3. Decomposition
Decomposers break down dead organic matter
Release CO2 back into the atmosphere
4. Combustion
Burning of fossil fuels releases CO2 into the atmosphere
Carbon Reservoirs
1. Atmosphere
Contains CO2 in the form of a greenhouse gas
2. Oceans
Absorb and store large amounts of CO2
3. Plants and Soils
Store carbon through photosynthesis and decomposition
4. Fossil Fuels
Coal, oil, and natural gas store carbon from ancient plant and animal remains
Human Impact on the Carbon Cycle
1. Deforestation
Reduces the ability of plants to absorb CO2
2. Burning Fossil Fuels
Increases CO2 levels in the atmosphere
3. Industrial Activities
Release large amounts of CO2 into the atmosphere
Impacts of Industrial Agriculture
Water Issues
Groundwater depletion from irrigation
Pollution from fertilizer and pesticide runoff
Sediment pollution from eroding soil particles
Pollution from animal wastes (livestock factories)
Enrichment of surface water from fertilizer runoff and livestock wastes
Air Pollution
Pesticide sprays
Soil particles from wind erosion
Odors from livestock factories
Greenhouse gases from the combustion of fossil fuels
Other air pollutants from the combustion of fossil fuels
Land Degradation
Soil erosion
Loss of soil fertility
Soil salinization
Soil pollution (pesticide residues)
Waterlogged soil from improper irrigation
Loss of Biological Diversity
Habitat fragmentation (clearing land and draining wetlands)
Monocultures (lack of diversity in croplands)
Stressors from air and water pollution
Stressors from pesticides
Replacement of many traditional crop and livestock varieties with just a few
Increases in Greenhouse Gases
Thermal Expansion of Seawater
Water expands as it heats up, and oceans have been absorbing most of the heat trapped by greenhouse gases released by fossil fuel burning, deforestation, and animal farming.
Example: If a container of seawater is heated, it will increase in volume as the temperature rises, demonstrating the principle of thermal expansion.
Effects: Warming waters caused as much sea level rise from 2002 through 2014 as the melting of all the glaciers and Greenland Antarctic ice sheets combined.
Climate Change Increases Diseases Transmitted Through Insects
Rising global temperatures can lengthen the season and increase the geographic range of disease-carrying insects.
Example: As temperatures warm, mosquitoes and other warm-weather vectors can move into higher altitudes and new regions farther from the equator
Effects: For instance, in some regions in the United States, warming is lengthening the season for Zika-carrying mosquitoes.
Increased rainfall, flooding, and humidity create more viable areas for vector breeding, allowing breeding to occur more quickly, as eggs hatch faster in hotter climates.
Cause and Effect: Officials braced for increased risk for Zika and West Nile virus infections after the massive flooding event in Louisiana in August 2016, which increased the breeding habits for Aedes mosquitoes.
Carbon Sequestration
Biological Carbon Sequestration
Biological carbon sequestration is the storage of carbon dioxide in vegetation such as grasslands or forests, as well as in soils and oceans
The most common/most known form of carbon sequestration
Geological Carbon Sequestration
Geological carbon sequestration is storing carbon dioxide in underground geologic formations, or rocks. Typically, carbon dioxide is captured from an industrial source, such as steel or cement production, or an energy-related source, such as a power plant or natural gas processing facility, and injected into porous rocks for long-term storage.
Happens naturally during the carbon cycle
Technological Carbon Sequestration
Scientists are exploring new ways to remove and store carbon from the atmosphere using innovative technologies. Researchers are also starting to look beyond the removal of carbon dioxide and are now looking at more ways it can be used as a resource.
![]()
Biodiversity
Biodiversity in an ecosystem includes genetic, species, and habitat diversity.
Population Bottleneck
The more genetically diverse a population is, the better it can respond to environmental stressers. Aditionally, a population bottleneck can lead to loss of biodiversity.
Population bottleneck is a sharp reduction in the size of a population due to environmental events or human activities, leading to a loss of genetic diversity.
 Biodiversity Importance
Biodiversity Importance
Diverse communities are generally more resilient to to disturbance than otherwise similar, but less diverse communities. A diverse community is one that would likely have overlaping niches. If one species is lost, another one may serve a similar role in the community.
Ecosystems with a larger number of species are more likely to recover from disrupters.
Habitat Loss
Without maintenance of very large blocks of inter-connected forest, there is a clear risk that hundreds of species could become extinct. Larger mammals such as elephants are particularly affected because of the vast, particular areas needed to survive.
Loss of habitat leads to a loss of specalist species, followed by a loss of generalist species. It also leads to reduced numbers of species that have territorial requirements.
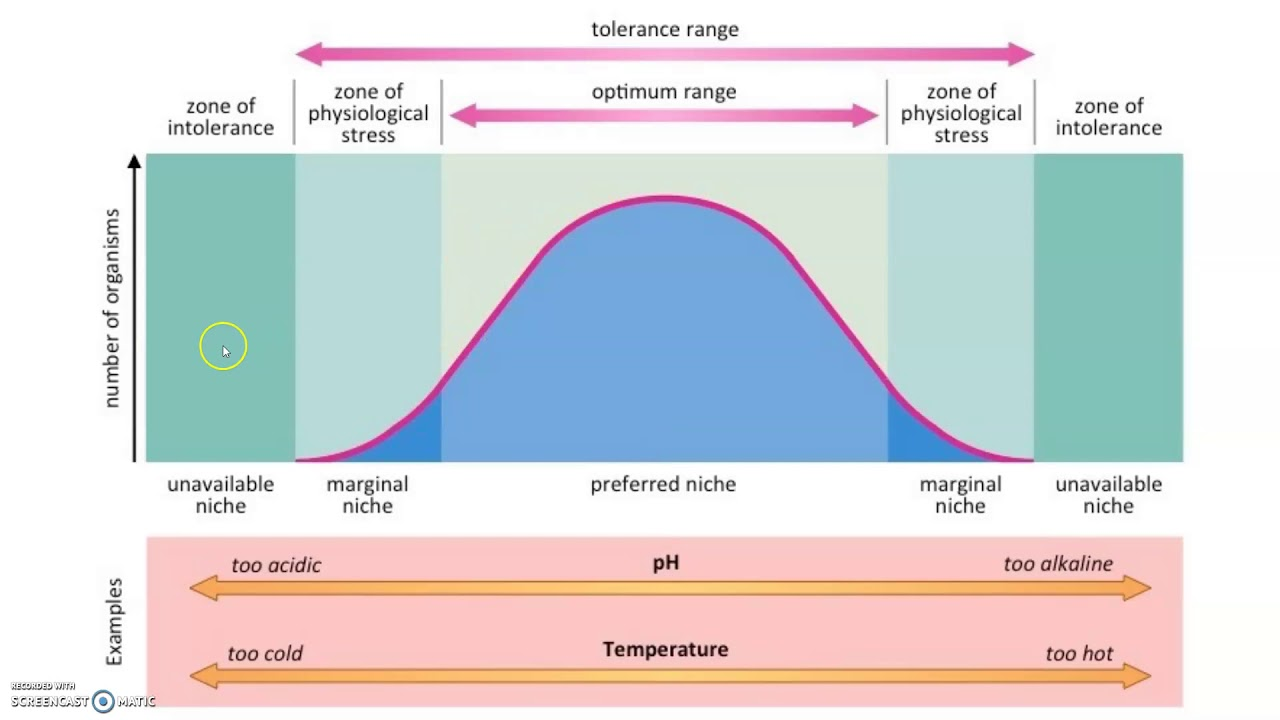
Ecological Tolerence
The range of conditions, such as temperature, salinity, flow rate, and sunlight an organism can endure before injury or death results.