Chapter 1 - Elements and the Periodic Table
Elements and the Periodic Table
Atomic Number
Definition: The number of protons in the nucleus of an atom.
Each element has a unique atomic number.
The periodic table is currently organized by increasing atomic number.
Atoms have an equal number of protons and electrons, resulting in no overall charge.
Ions are formed when atoms lose or gain electrons, acquiring a charge.
Atomic Structure
Element: A pure substance containing only one type of atom.
Atom: The smallest unit of matter.
Nucleus: The central region of an atom, containing protons and neutrons.
Proton: Positively charged particle within the nucleus.
Neutron: Neutral particle within the nucleus.
Electron: Negatively charged particle existing outside the nucleus.
Subatomic particle: Particles found within the atom (protons, neutrons, electrons).
Key Terms
Atomic number (n.): The number of protons in the nucleus of an atom.
Chemical symbol (n.): An abbreviation used to represent a chemical element.
Periodic table (n.): A table of chemical elements arranged by increasing atomic number.
Molecule (n.): Two or more atoms bonded by sharing electrons (e.g., H2).
Compound (n.): Two or more atoms of different elements bonded together (e.g., NH3).
Ion (n.): An atom that has gained or lost electrons, resulting in a charge.
Mass Number
Definition: Approximately equal to the sum of protons and neutrons in an atom.
Electrons have negligible mass, so they are not considered in mass number calculations.
Mass\ number = no.\ protons + no.\ neutrons
The mass number is a relative value and does not have units.
Calculating the number of neutrons:
Number\ of\ neutrons = mass\ number - atomic\ number
Isotopes
Definition: Variants of an element with the same atomic number but different numbers of neutrons.
All atoms of a specific element have the same number of protons. For example, all carbon atoms have 6 protons.
Isotopes of an element have different mass numbers (e.g., 12C, 13C, 14C).
Isotopic notation (scientific notation) is used to represent isotopes.
Periodic Table (Part 1)
The periodic table is an organizational tool to identify patterns and trends.
Metals, non-metals, and metalloids.
Electronic configurations (shell and subshell).
Atomic radii.
Relationships between structures and properties of elements. These properties includes: electronegativity, first ionisation energy, metallic and non-metallic character and reactivity.
Periods and Groups
Periods: Rows in the periodic table.
Groups: Columns in the periodic table.
Elements are arranged by increasing atomic number (number of protons).
Metals are generally on the left, non-metals on the right, and metalloids form a 'staircase' pattern.
Elements in the same group have similar reactivity.
Elements in the same period have the same number of electron shells.
Group 1 (Alkali Metals) and Group 17 (Halogens) are highly reactive. Group 18 (Noble Gases) are generally inert.
Electron Shells
Electrons are arranged in shells around the nucleus.
The first shell (closest to the nucleus) holds a maximum of 2 electrons.
Subsequent shells hold more electrons (8, 18, 32).
Electron configuration: Arrangement of electrons in shells.
Valence electrons: Electrons in the outermost shell.
Valence shell: The outermost energy shell containing valence electrons.
Energy shells: Orbits containing different levels of energy around the nucleus.
Electron shell notation: Summary of electrons per shell (e.g., 2, 8, 1).
Ground state: Electrons at their lowest possible energy level.
Blocks on the Periodic Table
Elements are categorized into blocks based on the location of their valence electrons.
s-block: Groups 1 and 2.
p-block: Groups 13 to 18.
d-block: Groups 3 to 12.
f-block: Lanthanoids (atomic number 57–71) and actinoids (atomic number 89–103).
Electronic Subshell Configuration
The Schrodinger model includes orbitals within each electron shell.
Orbitals: Regions with the highest probability of finding electrons.
The Pauli Exclusion Principle states that each orbital can hold 0, 1, or 2 electrons.
Electron subshell notation: Order of filling of sub-shells including s,p,d,f.
s subshell: 1 orbital, max. 2 electrons
p subshell: 3 orbitals, max. 6 electrons
d subshell: 5 orbitals, max. 10 electrons
f subshell: 7 orbitals, max. 14 electrons
Aufbau principle: Rule that states that subshells are filled by electrons from the lowest to the highest energy level. The filling order is typically:
1s < 2s < 2p < 3s < 3p < 4s < 3d < 4p < 5s < 4d < 5p < 6s < 4f < 5d < 6p < 7s ments like copper and chromium.
Core Charge
Definition: The effective pull of the positive nucleus on the valence electrons.
It increases across a period and is constant within a group.
Core\ charge = number\ of\ protons - inner\ shell\ electrons
Example: Lithium (Li) core charge = 3 - 2 = +1
Example: Potassium (K) core charge = 19 - 18 = +1
Atomic Radii
Definition: The distance from the center of an atom to the valence electrons.
Atomic radius decreases across a period (left to right) and increases down a group.
Core charge and the number of electron shells affect atomic radius.
Across a period, increasing core charge pulls valence electrons closer, reducing the radius.
Down a group, adding electron shells increases the atomic radius.
Periodic Table (Part 2)
Trends in properties affected by periodicity: atomic radius, electronegativity, first ionization energy, metallic character, and reactivity.
Periodicity: Characteristics of elements in a period.
Electronegativity
Definition: The ability of an element to attract shared electrons in a chemical bond.
Electronegativity increases across a period and up a group.
Electronegativity increases with:
Increasing core charge
Decreasing atomic radius
Fewer occupied electron shells
Fluorine is the most electronegative element; Noble gases aren’t considered.
First Ionization Energy
Definition: The energy required to remove the first valence electron from an atom.
First ionization energy increases across a period (due to increasing core charge and decreasing atomic radius).
First ionization energy decreases down a group (due to increasing number of electron shells and greater distance of valence electrons from the nucleus).
Metallic Character
The degree to which an element exhibits properties of a metal (luster, conductivity).
Metallic character decreases across a period and increases down a group.
Metals in groups 1 and 2 possess high metallic character (tendency to lose electrons).
Non-metals possess high non-metallic character.
Metalloids display a mixture of metallic and non-metallic properties.
Reactivity
The tendency of an atom to lose or gain electrons.
Noble gases (Group 18) are inert (unreactive).
Among metals, Group 1 is the most reactive, followed by Group 2.
Among nonmetals, Group 17 is the most reactive, followed by Group 16.
Reactivity trends depend on how easily electrons are lost or gained, atomic radius, core charge, and electronegativity.
Recycling Critical Elements
Critical elements: Elements in limited supply that could become depleted without recycling.
Recycling is important for element recovery and reducing waste.
Critical Elements
Help Protect PRAM:
Help - Helium
Protect - Phosphorus
P - Post-transition elements
R - Rare earth elements
A - And
M - Metalloids
Examples: Helium, phosphorus, rare earth elements (REE), post-transition metals, metalloids.
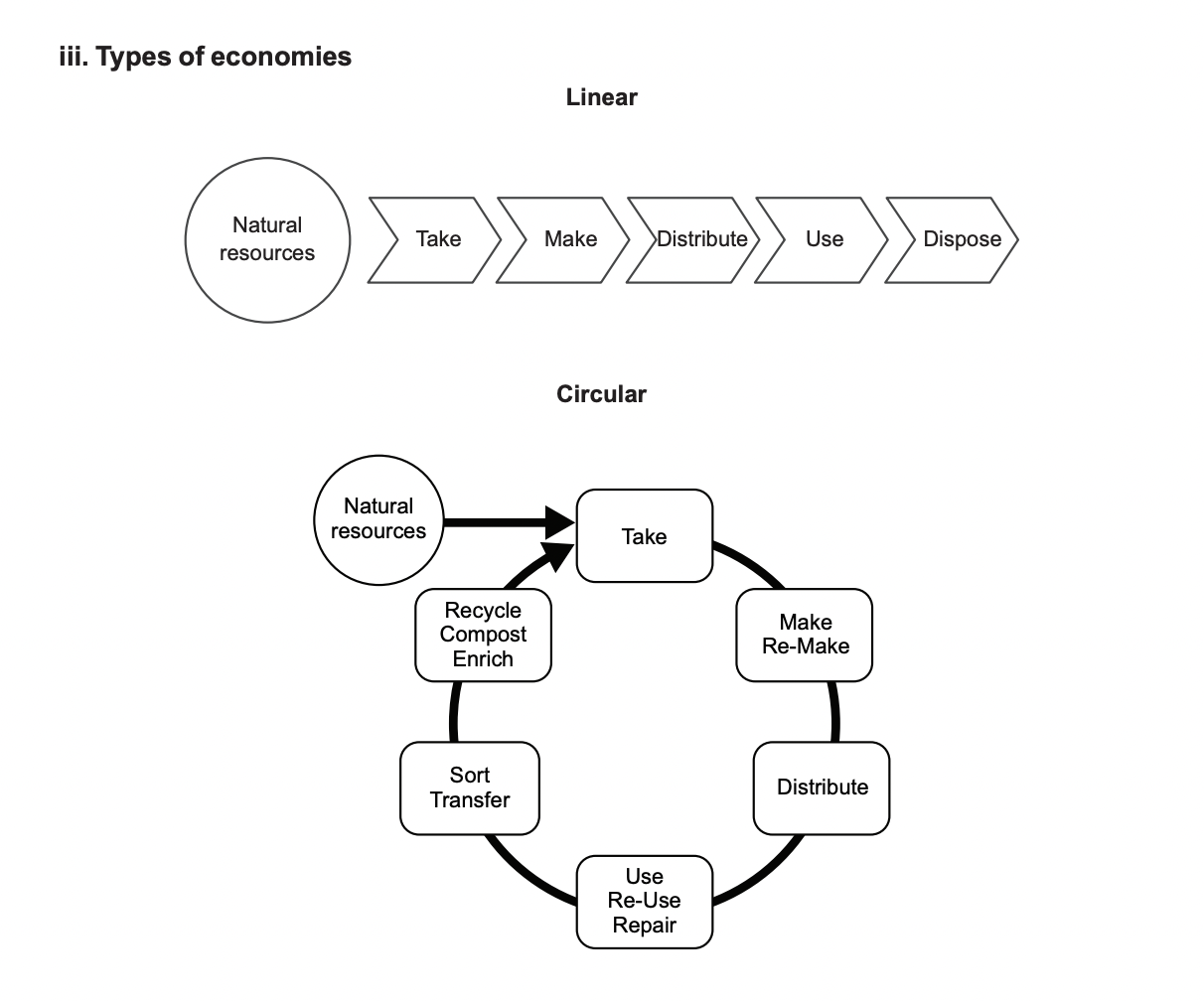
Transitioning to a Circular Economy
Sustainable development: Meeting present needs without compromising future generations.
Linear economy: A 'take-make-dispose' model.
Circular economy: A continuous cycle that focuses on the optimal use and re-use of resource from the extraction of raw materials through to the production of new materials.
Recycling technological waste (e.g., electronics) reduces landfill and increases the supply of critical elements.
This approach aligns with green chemistry principles, particularly waste prevention.
/