Topic 13: Innate Defense System
Overview of Host Resistance
- immune system
- composed of wide variety of cells, tissues, and organs
- recognizes foreign substances or microbes and acts to neutralize or destroy them
- “probiotic—up to 500 species”, “prebiotic” – up to ¾ immune system reside in your gut
- probiotic line intestine (mental health importance; dependent on nutrition)
- said will not ask # of species
- know difference between the two?
- compromised by stress, health problems & unhealthy food / lifestyle
- immunity
- ability of host to resist a particular disease or infection
- immunology
- science concerned with immune responses
Terminology
- Susceptibility: Lack of resistance to a disease
- Immunity: Ability to ward off disease
- Innate immunity: Defenses against any pathogen
- “nonspecific immunity”
- Adaptive immunity: Immunity, resistance to a specific pathogen
Types of immune responses
- Innate (nonspecific) defense system
- responds quickly, offers resistance to any microbe or foreign substance, lacks immunological memory, and consists of:
- First line of defense – skin and membranes
- Second line of defense – antimicrobial proteins, phagocytes, and other cells
- Inhibit spread of invaders throughout the body (stop the invaders)
- Inflammation is its hallmark and most important mechanism (cause of swelling, heat, redness)
- Where did you hear phagocytosis?
- WBC will “eat” them; bacterias with capsule resistant to phagocytosis
Immunity
- Innate Immunity
- First Line of Defense
- Intact skin
- Mucous membranes and their secretions
- Normal Microbiota (antagonism)
- antagonism is your normal bacteria on skin that keeps you “clean” → will be “mean/antagonize” new bacteria
- Second Line of Defense
- Natural Killer cells and phagocytic WBC
- Inflammation
- Fever
- Antimicrobial substances
- Adaptive Immunity (“specific”)
- Third Line of Defense
- Specialized lymphocytes: T and B cells (T cells are HIV’s target; B cells give antibodies)
- Antibodies
- received by getting sick and producing B cells or getting vaccine with B cells
Adaptive (specific) defense system
- Also called acquired or induced immunity, has immunological memory, responds to a very particular foreign substance (why some substances last a shorter time than others? they don’t know yet; COVID is one that doesn’t have a long memory)
- about 2 weeks to produce antibodies (don’t get vaccine when people are already sick, do it sooner)
- Third line of defense
- Takes longer to react than the innate system
- Works in conjunction with the innate system
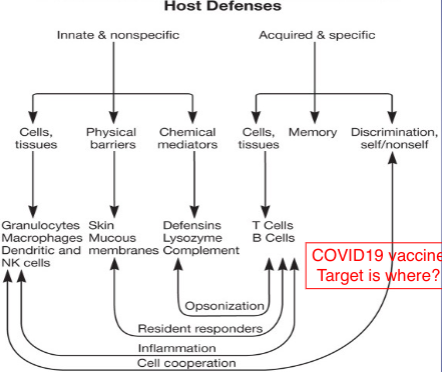
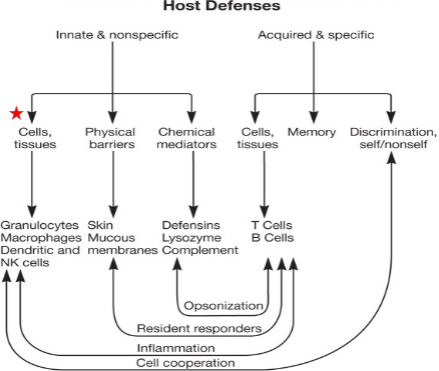
Components of the Innate Immune System
- Skin (biggest organ)
- Mucous
- most pathogens go through mucous membrane (eyes, nose, mouth)
- Covid going for respiratory; cytocines? caused continous inflammation
- Chemical
- stomach acid
- food poisiong indicates eating a lot of bacteria
- bacteria can go up through urinary tract and cause UTI, if not treated the bacteria can travel upwards
- lysozyme cuts galasidic bond?
- smokers cough in morning because paralysis of cilia (cilia moves the fluid upwards)
Innate (non-specific) defense systems
- Surface Barriers: Skin, mucous membranes, and their secretions make up the first line of defense
- sebaceous glands → oils (sebum)
Skin
- Largest organ (20 sqft), 10+/- pounds
- strong mechanical barrier to microbial invasion
- keratin produced by keratinocytes (=basal cells) in outer layer
- resists absorption of water and most inorganic chemicals; allows absorption of many organic and a few inorganic chemicals
Skin infection/reaction by microbes
- Cellulitis: inflammation due to infection
- does not have to be an open cut
- Warts: viral infection cause excess skin growth
- Herpes: HSV-1&HSV-2, periodic blisters around lips or genitals
- cold sores
- Hives: allergic reaction – not infection
- Tinea: skin mycosis
- fungal skin infection
- Shingles: varicella zoster virus (linear DNA, lipid enveloped, herpes group)
- DNA virus, enveloped, hide when young but “come out” when older
- younger people can get it as well
Skin = inhospitable environment for many microbes
- attached organisms removed by shedding of outer skin cells = part of your soap scum, eww
- pH 3-5 = acidic
- high NaCl concentration = why?
- skin bacteria have a high salt toleration and dryness (mannitol salt agar!)
- subject to periodic drying
- Lysozyme in saliva and tears – function
- prevents infection
- Fungistatic fatty acids in sebum
- Transferrin** in blood (who’s the bad guy?)
- *Antagonisms: competitive exclusion of normal microbiota (our bacteria)
- **iron-binding blood glycoproteins
More about Skin
- specialized cells called skin-associated lymphoid tissue (SALT)
- Langerhans cell---NOT islet of Langerhans in pancreas!!!
- dendritic cell that can phagocytose antigens
- have lots of branches; can eat the pathogens (bring inside cell)
- differentiates into interdigitating dendritic cell–presents antigen to and activates T cells
- uses piece of pathogen to present to T cell
Antimicrobial Secretions
- lysozyme
- How?: tears, saliva
- cut 1-4 galoscidic bond
- lactoperoxidase
- produces superoxide radicals: toxic
- mammary and salivary gland (saliva)
The Eye
- flushing action of tears
- lysozyme, lactoferrin and secretory IgA in tears
- lactoferrin - transferrin (good?)
- Lactoferrin: multifunctional protein (antimicrobial)
- IgA = antibody
- cover antibodies later
Mucous Membranes
- form protective covering that resists penetration and traps many microbes
- are often bathed in antimicrobial secretions which contain a variety of antimicrobial substances
- contain mucosal-associated lymphoid tissue (MALT)
- mucous can trap bacteria
Mucosal-Associated Lymphoid Tissue (MALT)
- specialized immune barrier
- gut-associated lymphoid tissue (GALT)
- bronchial-associated lymphoid tissue (BALT)
- two types of MALT
Respiratory system
- turbulent air flow deposits microbes onto mucosal surfaces
- COVID 19 TARGET
- Mucociliary blanket
- mucous secretions that traps microbes
- once trapped, microbes transported away from the lungs (mucociliary escalator)
- can be expelled by coughing or sneezing
- salivation washes microbes to stomach (pH 3-5)
- alveolar macrophages
- phagocytic cells in alveoli of lungs
- capsule bacteria prevent digestion by phagotcytic cells
When you smoke…
- Cilia paralized, smoker’s cough
- being moved upwards
- Smokers are sick more often because……
- cilia is paralized therefore cilia isn’t moving upwards
- Morning cough
- 80% lung cancer – due to smoking, 13% survive 5+ years
- includes 2nd hand smoking
- P53 gene – nose, liver, colon, myloid leukemia
- cancer suppressing gene
- Tobacco smoke contains a deadly mix of more than 7,000chemicals. Hundreds are toxic. About 70 can cause cancer. Here are some of the chemicals. (said wouldn’t ask about chemicals, just information)
- Cancer-Causing Chemicals
- Formaldehyde: Used to embalm dead bodies
- Benzene: Found in gasoline
- Polonium 210: Radioactive and very toxic
- Vinyl chloride: Used to make pipes
- Toxic Metals
- Chromium: Used to make steel
- Arsenic: Used in pesticides
- Lead: Once used in paint
- Cadmium: Used to make batteries
- Poison Gases
- Carbon monoxide: Found in car exhausts
- Hydrogen cyanide: Used in chemical weapons
- Ammonia: Used in household cleaners
- Butane: Used in lighter fluid
- Toluene: Found in paint thinners
Helicobacter pylori –in the disease packet
- Gram -, Curved rod, Microaerophilic
- microaerophilic - likes less oxygen (strept throat test)
- 80% of infected people = asymptomatic
- Gastritis, linked to duodenal and stomach cancer – stress was to blame before the discovery
- burrow into stomach
- high salt diet dissolves membrane in stomach (high salt diet = higher chance of stomach cancer)
- Stomach acid gradient chemotaxis
- urea in stomach acid
- Urease –Ammonia production, ph?
- metabolize protein, pH increases
- 1st infection – antibody test
- 2nd and after – Urea or stool test
- because possible antibodies from last infection
 Picture: blood has plasma and cells (red blood cells, platelets, and white blood cells); centrifuge separates layers
Picture: blood has plasma and cells (red blood cells, platelets, and white blood cells); centrifuge separates layersBlood Plasma – approx. 55%
- Glucose, fat
- Protein – (antibodies 1/3)
- Clotting factor
- Electrolytes, vitamins
- Hormones
- BP, pH
- less fluid increase BP; neutral pH
- CO2
Donations
- Blood donation ---- NO NO
- Have tested positive for hepatitis B or hepatitis C, lived with or had sexual contact in the past 12 months with anyone who has hepatitis B or symptomatic hepatitis C.
- After donation, test for ….HIV, hepatitis, syphilis, Human T-lymphotropic virus
- Platelets donation – not from mama. Why????
- pregnant - may have antibody from baby
- Plasma donation – no tuberculosis, malaria, sickle cell anemia, cancer etc..
- screening
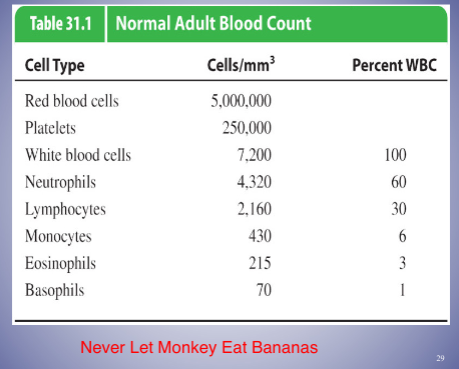
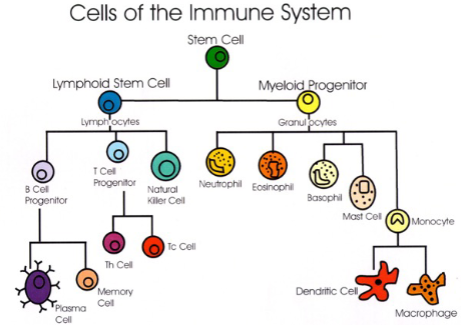
White Blood Cells and the Nonspecific and Specific Responses
- white blood cells (WBCs) - major role in the innate and specific responses
- Hematopoesis – hematopoetic stem cell differentiation process (all blood components)
- stem cells that differenate
- umblitical cord has stem cells
- development of white blood cells in bone marrow of mammals
- WBCs that mature prior to leaving bone marrow, e.g. macrophages and dendritic cells, become part of innate immune system and will respond to all antigens
- WBCs that are not fully functional after leaving bone marrow become part of the adaptive immune response, e.g.B and T cells and could differentiate in response to specific antigens
- know the differences
Monocytes and macrophages
- highly phagocytic cells, 6% of WBC
- engulf pathogen, lysosome digests pathogen
- make up monocyte-macrophage system
- monocytes
- are mononuclear phagocytic leukocytes
- after circulating for ~8 hours, mature into macrophages
- macrophages
- reside in specific tissues
- have a variety of surface receptors
- senses the pathogens
- named according to tissue in which they reside
Dendritic Cells: Antigen-presenting cells (APC)
- present in small numbers in blood, skin, and mucous membranes of nose, lungs, and intestines
- contact, phagocytose and process antigens → display foreign antigens on their surfaces (antigen presentation)
- bring antigen/pathogen to surface to show other cells (i.e macrophages)
Basophils
- stain bluish-black with basic dyes, 1% of WBC
- Non-phagocytic
- release histamine, heparin, prostaglandins, serotonin, and leukotrienes from granules
- histamine most important
- play important role in development of allergies and hypersensitivities (inflammation)
- antihistamines
Eosinophils
- stain red with acidic dyes, 3% of WBC
- defend against parasites (protozoan and helminthes)
- play a role in asthma/allergic reactions along with mast cells
Neutrophils
- stain at neutral pH
- 60% of WBC - majority
- highly phagocytic - 1st to go to site
- circulate in blood then migrate to sites of tissue damage
- sequeeze through capillary walls
- kill ingested microbes with lytic enzymes and reactive oxygen metabolites
- high neutrophil count = bacterial infection
- pus is normally dead neutrophils
Mast Cells
- differentiate in blood and connective tissue
- contain granules containing histamine, heparin, and other pharmacologically active chemicals, over 200+ chemicals
- play important role in development of allergies and hypersensitivities
- Mast cell activation syndrome
- idopathic - don’t know what it is, may be genetic
Lymphocytes
- major cells of the immune system, 30% of WBC
- major populations include T cells, B cells, and natural killer (NK) cells
- B and T lymphocytes differentiate in bone marrow from stem cells
B Lymphocytes
- B cells (B lymphocytes)
- mature mostly in lymph nodes and other lymph tissues
- circulate in blood
- can settle in lymphoid organs
- after maturation and activation are called plasma cells and produce antibodies
- memory and antibodies (after ~10 days)
- outside of pathogens
T Lymphocytes
- T cells (T lymphocytes)
- Mature primarily in the thymus gland
- can remain in thymus, circulate in blood, or reside in lymphoid tissue
- like B cells, require antigen binding to surface receptors for activation and continuation of replication
- need a signal (i.e antigen presenting cell - dendritic cell)
- they have no memory or antibodies
- cytokines, chemicals that have effects on other cells, are produced and secreted by activated T cells
Natural Killer (NK) Cells
- small population of large non-phagocytic granular lymphocytes
- kill malignant cells and cells infected with pathogens
- two ways of recognizing target cells
- bind to antibodies which coat infected or malignant cells (antibody-dependent cell-mediated cytotoxicity (ADCC))
- recognizes cells that have lost their class I major histocompatibility (MHC) antigen due to presence of virus or cancer
- organ transplant
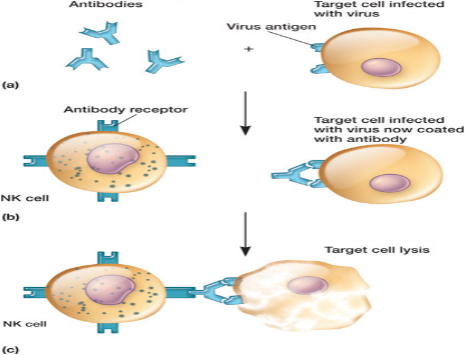
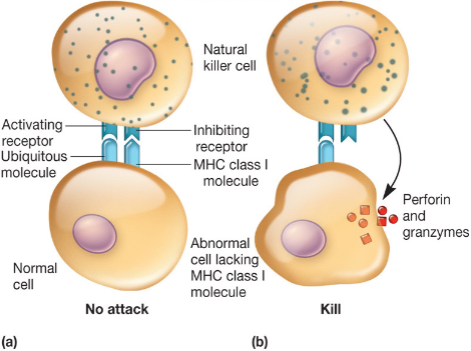
Cytotoxic T Cells and Natural Killer Cells
- Cytotoxic T-cells : the specific antigens presented by their MHC class I molecule
- recognize receptor and present it
- NK cells : the absence of MHC class I molecules, specific types of antibodies, and \n some type of cellular stress
- Know the difference!
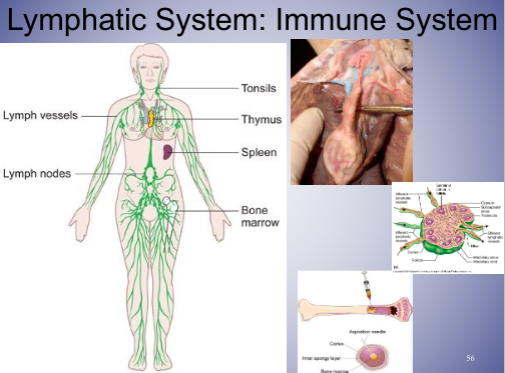
Primary Lymphoid Organs and Tissues
- immature undifferentiated lymphocytes (generated in the bone marrow) → mature
- obtain a specific antigenic specificity within the primary lymphoid organs and tissues, bone marrow and thymus gland
- unique to pathogens
Secondary Lymphoid Organ/Tissue
- Secondary lymphoid tissue includes: lymph nodes, tonsils, adenoids, Peyer’s patches (intestine), spleen
- throughout the body
- interface between innate and acquired host immunity (overlap)
- act as areas of antigen sampling and processing
- determine if the threat needs to be neutralized
- some lymphoid cells are found closely associated with specific tissues
- e.g., skin-associated lymphoid tissue (SALT)
- e.g., mucous-associated lymphoid tissue (MALT)
- e.g. bronchial associated lymphoid tissue (BALT)
Secondary Lymphoid Organ/Tissue
- spleen
- highly organized lymphoid organ
- filters blood - scanning
- trap microbes and antigens
- present antigens to B and T cells
- most common way that lymphocytes become activated to carry out their immune functions
- lymph nodes
- highly organized lymphoid tissue
- filter lymph
- microbes and antigens trapped and phagocytosed by macrophages and dendritic cells
- B cells differentiate into memory and plasma cells within lymph nodes
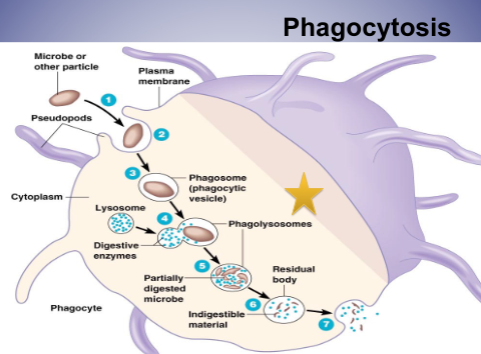
Phagocytosis
- process by which phagocytic cells (monocytes, tissue macrophages, dendritic cells and neutrophils) recognize, ingest and kill extracellular microbes
- How bacteria resist?
- capsule
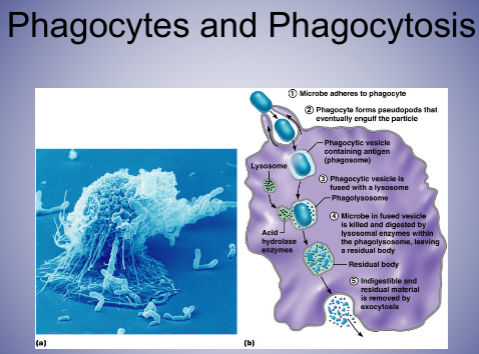
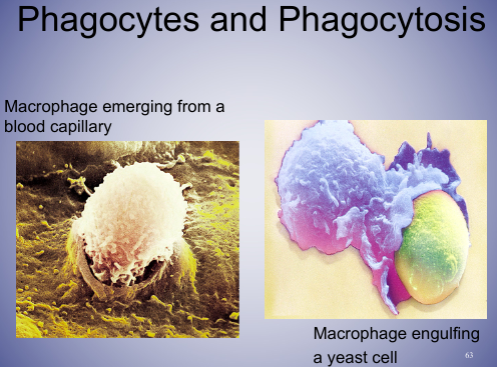
Phagocytosis
- two mechanisms for recognition of microbe by phagocyte
- opsonin-independent (nonopsonic) recognition
- opsonin-dependent (opsonic) recognition
- phagocytosis can be greatly increased by opsonization
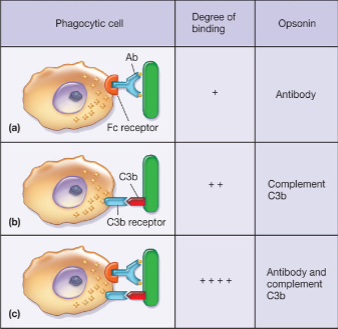
Opsonization (stopped here w/ anki)
- opsonin – Greek: prepare for eating
- opson – Greek: delicious side dish
- process in which microbes are coated by serum components in preparation for recognition/ingestion by phagocytic cells
- molecules that carry out above are called opsonins = antibodies, complement molecules
- some complement proteins are opsonins
- bind to microbial cells, coating them for phagocyte recognition
Opsonin-Independent Mechanism
- involves nonspecific and specific receptors on phagocytic cells
- four main forms:
- recognition by lectin-carbohydrate interactions
- recognition by protein-protein interactions (PPI)
- recognition by hydrophobic interactions
- detection of pathogen-associated molecular patterns (PAMPs) by pattern recognition receptors (PRRs, e.g., toll-like receptors)
- *lectin: carbohydrate binding proteins
- *PPI: Alzheimer’s, CJD, Cancer
Pathogen-Associated Molecular Patterns (PAMPs)
- PAMPs are unique to microbes, not present in host
- Examples of unqiue features
- e.g., lipopolysaccharide (LPS) of gram negative bacteria
- e.g., peptidoglycan of gram positive bacteria
- PAMPs recognized by pattern recognition receptors (PRRs) on phagocytic cells
Toll-Like Receptors (TLRs)
- recognize and bind unique PAMPs of viruses, bacteria or fungi
- Innate
- Macrophages, Dendritic cells
- on these cells
Intracellular Digestion
- phagolysosome
- vacuole which results from fusion of phagosome with lysosome
- presence of toxic chemicals
- e.g., degradative enzymes
- e.g., toxic reactive oxygen intermediates (ROIs) (kills microorganisms)
- e.g., reactive nitrogen intermediates (RNIs)
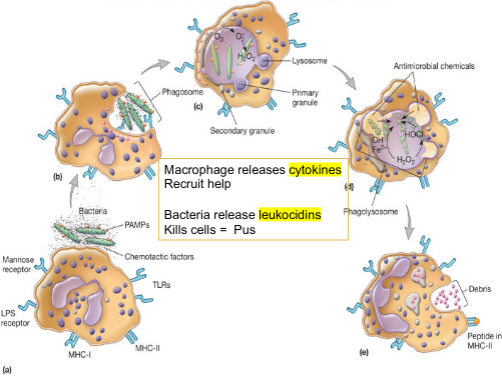
Neutrophils – after digesting microbial fragments
- also phagocytic - 1st to be at site of injury
- Exocytosis
- process used by neutrophils to expel microbial fragments after they have been digested
- phagolysosome unites with cell membrane
- results in extracellular release of microbial fragments
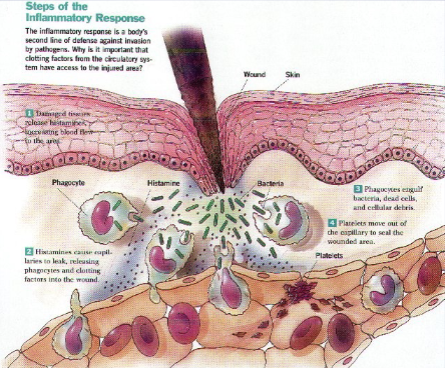
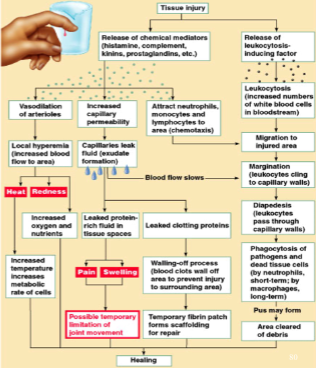
Inflammation (innate side)
- nonspecific response to tissue injury
- can be caused by pathogen or physical trauma
- acute inflammation is the immediate response of body to injury or cell death
- cardinal signs---PRISH (reactions from inflammation)
- Pain – release of chemicals such as histamine
- Redness – increased blood flow
- Immobility - altered or loss of function
- Swelling – edema (accumulation of fluid)
- application of ice pack (no more than 20 min because it slows the process of blood flow which gets rid of the waste)
- Heat – increased blood flow
Acute Inflammatory Response
- Vascular phase first, then cellular phase
- vascular is the fluid
- the release of inflammatory mediators from injured tissue cells initiates a cascade of events which result in the signs of inflammation
- involves chemical mediators
- chemokines - signaling proteins/cytokines
- released by injured cells
- selectins
- cell adhesion molecules on activated capillary endothelial cells
- integrins
- adhesion receptors on neutrophils
- blood vessel will get loose because of histamine and neutrophils can squeeze through
Inflammatory Response Vascular Permeability
- Vasodilation
- Chemicals released by the inflammatory response stimulate mast cells next to capillaries
- Mast cells release histamines to increase permeability of capillaries
- histamines make you “leaky”
- Plasma seeps into tissue (interstitial) spaces causing local edema (swelling), which contributes to the sensation of pain
- *pain – Na+channel
- lidocaine blocks Na+ channel
Inflammatory Response Phagocytic Mobilization
- Margination – neutrophils cling to the walls of capillaries in the injured area
- Diapedesis – neutrophils squeeze through capillary walls and begin phagocytosis
- know margination and diapedesis
- Chemotaxis – inflammatory chemicals attract neutrophils to the injury site
Chronic Inflammation
- slow process
- may not notice
- rhuematoid arthrisis
- excema
- involves formation of new connective tissue
- usually causes permanent tissue damage
- dense infiltration of lymphocytes and macrophages at site of inflammation
- granuloma
- walled off area formed when phagocytic cells can’t destroy pathogen
~~Opsonization~~
- ~~process in which microbes are coated by serum components in preparation for recognition/ingestion by phagocytic cells~~
- ~~molecules that carry out above are called opsonins~~
- ~~make pathogen more visible~~
- ~~some complement proteins are opsonins~~
- ~~bind to microbial cells, coating them for phagocyte recognition~~
Pus
- Dead leukocytes (mostly neutrophils)
- Color varies
- Abscess=enclosed in tissue
- Pimple=visible collection within/beneath the epidermis
- Pus causing bacteria = pyogenic
- Example from your lab: Staphylococcus aureus (pink eye), S. epidermidis, S. pyogenes (strept throat) (Gram+, β-hemolysis, catalase-), Escherichia coli, Pseudomonas aeruginosa
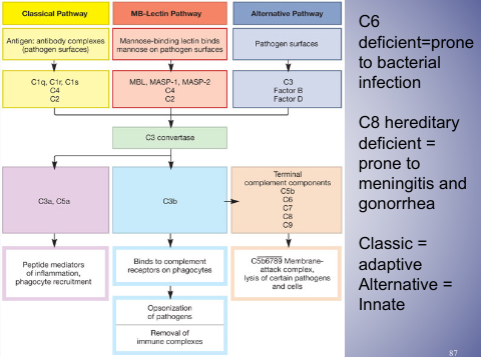
The Complement System (or cascade)
- composed of >30 serum proteins – mainly produced in liver (pro-proteins)
- augments (or “complements”) the antibacterial activity of antibody
- part of innate immunity, will NOT change over ones lifetime, does not adaptable
- genetic, pre-determined
- aide in getting rid of pathogen
Other Functions of Complement Proteins
- function as chemotactic signals that recruit phagocytes to their activation site
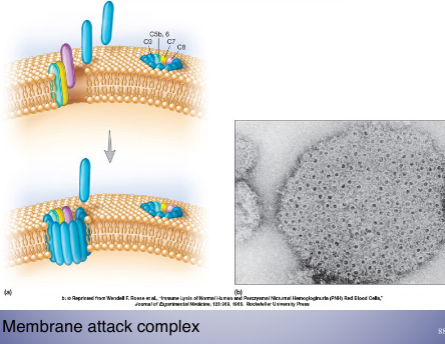
- puncture cell membranes causing cell lysis
- important function
- many complement activities unite the nonspecific and specific arms of the immune system to destroy and remove invading pathogens
Complement Activation Pathways (innate)
- specific proteins are unique to the first part of each of the three complement activation pathways, but all complement pathways have the same outcome
- Opsonization - phagocytosis
- stimulation of inflammatory mediators
- lysis of microbes by membrane attack
- all pathways are activated as a cascade; the activation of one protein results in the activation of the next
- all complement proteins are in the inactive state until activation when the host is challenged by an invading microbe
Cytokines
- soluble proteins or glycoproteins that are released by one cell population that act as intercellular mediators or signaling molecules
- monokines
- released from mononuclear phagocytes
- i.e macrophages
- lymphokines
- released from T lymphocytes
- interleukins
- released from one leukocyte and act on another leukocyte
- colony stimulating factors (CSFs)
- act on hemopoietic stem cell, stimulate growth and differentiation of immature leukocytes in bone marrow
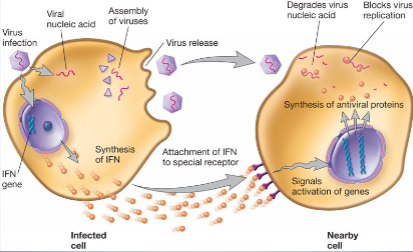
Interferons (IFNs) =type of cytokines
- regulatory cytokines produced by some eukaryotic cells in response to viral infection
- viral infection is important (acute)
- do not prevent virus entry into host cells, but defend against viruses by preventing viral replication and assembly
- also help to regulate the immune response
- responsible for “flu-like” symptoms
- clinical use for viral infection, MS and cancer treatment
- cancer treatment: elicit T cells (side effects: thinning hair, flu-like symptoms); T cells attack cancer
Fever
- 37.5-38.3 °C (99.5-100.9°F) or above
- dr starts to get worried at 105
- most common cause of fever is viral or bacterial infection or bacterial toxins
- Viral --- DO NOT ask for antibiotics!!!!
- Thermostat set point located in hypothalamus
More About Fever
- in most cases, the endogenous pyrogen, a cytokine produced in response to pathogen, directly triggers fever production
- after release, pyrogens → hypothalamus and induce production of prostaglandins which reset hypothalamus to a higher temperature
- increase temp
- When the hypothalamus is reset, what has to happen to increase body temperature?
- *Pyrogen = a fever inducing substance
- **Prostaglandins = found in every tissue, hormone-like effect, lipid derived
- ***Physical activity is needed to increase metabolic rate, heat production = This accomplished by shivering thermogenesis.
- know where body’s thermostat is
Should fever be reduced with medicines?
- Yes! Because……
- Febrile seizure (epileptic seizure) – can be dangerous
- some people can get seizures from fever (temperature increases too quickly)
- Feeling awful/miserable – treating the symptom, not the cause
- fever caused by infection
- bacterial infection treated by antibiotics, no treatment for viral infection
- No! because……….
- Not high enough fever
- may hinder immune system
- Research (2014) has shown that using fever-suppressing drugs may allow patients to mistakenly feel better quicker than normal resulting in their premature return to the population
- Concerning influenza, it is estimated that this will result in a 1% increase in the number of cases and about 700 more deaths each year in the U.S.
- contributes to spread