ap bio : unit 1 cram sheet
Author Notes
hello!! welcome to my unit 1 cram sheet of the first unit; chemistry of life!!! majority of my notes r derived frm the ap daily videos and my teacher lectures so yeah!!! enjoy enjoy <33
like this cram sheet? check out my other cram sheets!!!
external resources that are similar to a section will also be linked at the end!
╭ Other Resources :
:: cararra ﹒﹒ 25 min ap bio review video based from the campbell biology 11th edition textbook!
:: sticky science﹒﹒short, bite-sized review videos in the form of reels from a previous ap bio student who got a 5 on the AP exam!
:: the APsolute recAP﹒﹒22 min review video based on all the topics of unit 1!
:: khan academy ﹒﹒the entire unit 1 course from khan academy!!
:: fiveable ﹒﹒ reviews unit 1 with articles and quizzes for you to practice your knowledge on!
﹙✦﹚﹒﹒abbreviations are used throughout <3
NOTE: for some reason some imgs won’t show up :(( all images will show up on mobile tho :)
﹙1.1 - Structure of Water and Hydrogen Bonding﹚
✦﹒water is composed of oxygen and hydrogen
the two form a covalent bond, where oxygen and hydrogen share their electrons
oxygen and hydrogen are specifically polar-covalent, as of the polarity from the differences in electronegativities
oxygen is more electronegative than hydrogen
water has a hydrogen bond, which is a weak bond interaction btwn negative and positive molecules
polar molecules are hydrophilic (water-loving)
non-polar molecules are hydrophobic (water-hating)
an example of this includes phospholipids, which are a part of the plasma membrane
✦﹒water has many special properties—
cohesion is caused by hydrogen bonds btwn molecules of the same type
this enables water to have surface tension enables small objects to “float” on water (ex: water striders r allowed to stand on water thanks to surface tension)
cohesiveness allows ice to be less dense than liquid water and therefore float on water
adhesion is caused by hydrogen bonds btwn different types of molecules
adhesiveness makes water a universal solvent, being able to dissolve a lot of substances in its liquid state
has a high heat capacity the provides thermal stability
caused frm water’s cohesive property, being able to absorb a lot of thermal energy before changing chemical states, resisting sudden temp changes
has high latent heat of vaporization, meaning it’ll take a lot of energy to change phases
example is human’s sweat; evaporation of sweat leaves a cooling effect thanks to this
has capillary action, in which water has the ability to move thru narrow spaces due to the forces of adhesion, cohesion, and surface tension
movement of water going up is against gravity
example - plants use this action ot bring water up frm the roots to the stem
╭ Other Resources :
:: amoeba sisters ﹒﹒ in-depth on the properties of water !!
﹙✦﹚﹒grasping the concept of water’s properties is ideal! be able to provide an example with each too :)
﹙1.2 - Elements of Life﹚
✦﹒living systems req. a constant input of energy
first law of thermodynamics/ law of conservation of energy states that energy cannot be created or destroyed; only transformed
living systems follow this law along w/other laws of energy
energy is usually used frm the energy stored in chemical bonds
✦﹒living systems req. a exchange of matter
atoms + molecules frm the environment r necessary to new new molecules, including—
carbon, used to build ALL macromolecules, store energy + form cells
its need of 4 bonds to complete its valence shell give it great versatility
nitrogen, used to build nucleic acids + proteins
phosphorus, used to build nucleic acids and certain lipids
﹙1.3 - Intro to Biological Macromolecules﹚
✦﹒monomers make polymers
all macromolecules are polymers
ALL monomers have carbon
polymers are specific to the monomers they consist of
monomers result from hydrolysis
this is where polymers are hydrolyzed (broken down) into monomers during the reaction
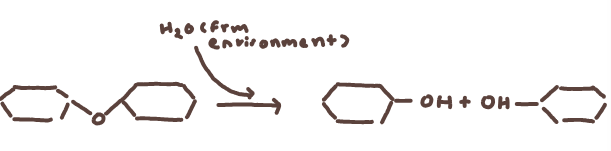 hydrolysis reaction where maltose (polymer) with water from the environment breaks down into two glucose molecules (monomers)
hydrolysis reaction where maltose (polymer) with water from the environment breaks down into two glucose molecules (monomers)polymers result from dehydration synthesis
oxygen and two hydrogen molecules from the monomers will break off to form water and there the monomers will form a covalent bond together becoming a polymer
this reaction consumes energy
 reaction where two glucose molecules (monomers) form maltose (polymer) with the addition of water
reaction where two glucose molecules (monomers) form maltose (polymer) with the addition of water
﹙✦﹚﹒ my teacher said that dehydration synthesis “comes up a lot” so best if you understand this reaction :)
﹙Carbohydrates & Lipids﹚
✦﹒carbohydrates are composed of linear chains of sugar monomers connected by covalent bonds
chains may be linear or branched
small direction of change can change function
made up of CH2O in a 1:2:1 ratio
✦﹒saccharides are carbs that consist of sugar molecules
monosaccharides r composed of one sugar tht provide a quick energy source
ex: ribose
monomer of carbohydrates
disaccharides r double sugars composed of double sugars
ex: matrum, lactose
polysaccharides r polymers composed of many sugars that are joined together by dehydration reactions
cellulose is a structural polysach. that helps build strong exoskeletons within organisms
starch is a storage polysach. that stores energy; plants store this, while animals store glycogen
has a curved shape, easily broken down
✦﹒lipids is a nonpolar chain that mixes poorly w/water due to structure
examples include the various fats (triglyceride, fatty acid)
they r large molecules assembled by smaller molecules via dehydration reactions
fatty acids work as long-term energy storage
can be saturated, which turns solid at room temp.
can be unsaturated
another example includes phospholipids

has 2 fatty acids instead of 3
in water, forms a bilayer to shield the tails frm water
﹙Proteins﹚
✦﹒proteins are made up of amino acids
amino acids have directionality with an amino terminus/group and carboxyl terminus/group
amino acids r added to the carboxyl grp of a growing peptide chain by formation of covalent bonds
amino acids are connected together by peptide bonds
together, amino acids form a polypeptide chain (polymer of amino acids)
the r-group of the amino acid is the only thing that differs btwn each amino acid; its how each amino acid is different from each other
 common structure of amino acids
common structure of amino acids
✦﹒proteins have different levels of structures
primary structure is a linear chain sequence of amino acids held together by peptide bonds
the last thing remaining when a protein denatures
secondary structure is created btwn the carboxyl grp and amino grp
can fold as a alpha helix shape or beta pleated sheet

formed frm the product of primary structure
stabilized by hydrogen bonds of primary structure
tertiary structure is the overall 3D shape of the protein composed of many interactions btwn the side chains/groups of various amino acids; these bonds stabilize the protein
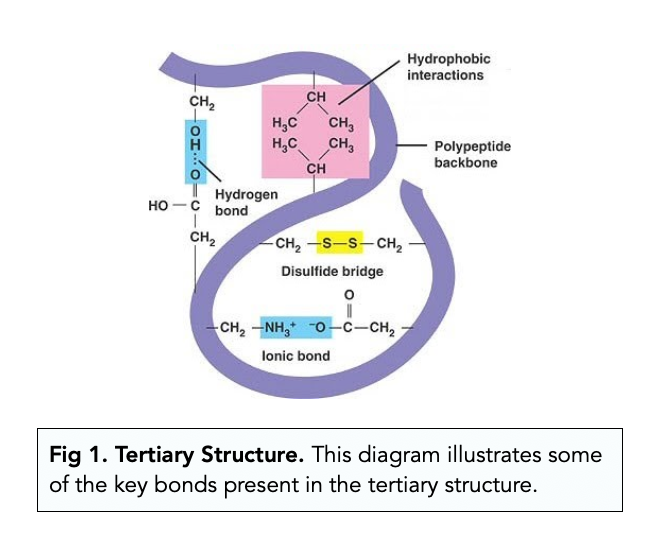
amino acids may have a charge— negative and positive charges will bond together and create an ionic bond on the protein
hydrogen bonds will form between polar r groups
disulfide bridge will be formed between two cysteine amino acids
hydrophobic interactions occur when nonpolaramino acids fold inwardsto hide itself from water (nonpolar = hydrophobic)
hydrophilic interactions occur when polaramino acids fold outwardstowards the water (polar = hydrophilic)
quaternary structure is the overall protein structure that is frm aggregation of polypeptide subunits
╭ Other Resources :
:: amoeba sisters ﹒﹒ video on protein structure and folding
﹙✦﹚﹒the structures of protein is very important to understand, especially what happens if one fails
﹙Nucleic Acids﹚
✦﹒r polymers (can be DNA or RNA)made up of monomers called nucleotides

a nitrogenous base is classified as a
pyrimidine (uranine [RNA only], cytosine, thymine), consisting of a six-membered ring of carbon and nitrogen
purine (adenine, guanine) , consisting of a six-membered ring fused w/a five-membered ring
╭ Other Resources :
:: amoeba sisters ﹒﹒ a video on ALL the biomolecules (carbs, lipids, proteins, nucleic acids)
 Knowt
Knowt
