ap bio : unit 3 cram sheet
Author Notes
hello hello! thank you for visiting my unit 3 cram sheet on cellular energetics !! all info in this cram sheet is derived from the AP daily videos and some of my teacher lecture notes :)
like my cram sheets? check out my others via profile <3 (all units will be done b4 exam??!)
external resources tht r similar to a section will be linked at the end!!!!! gl studying!!!
╭ Other Resources :
:: cararra ﹒﹒ 23 min ap bio review video based from the campbell biology 11th edition textbook!
:: sticky science﹒﹒short, bite-sized review videos in the form of reels from a previous ap bio student who got a 5 on the AP exam!
:: khan academy ﹒﹒the entire unit 3 course from khan academy!!
:: fiveable ﹒﹒ reviews unit 3 with articles and quizzes for you to practice your knowledge on!
﹙✦﹚﹒﹒abbreviations r used throughout this sheet :]
﹙3.1-3.3 - Enzymes﹚
✦﹒enzymes are biological catalysts that speed up biochemical reactions
enzymes often end in -ase (catalase, sucrase, lactase, amylase )
has an active site that specifically interacts with a specific substrate (like a lock and key)
this initiates a reaction (synthesis or digestion of substrate)
changes in shape causes denaturation
active site can no longer bind to specific substrate
denaturation can be reversible in some cases
✦﹒every enzyme has a optimum range
range in which reaction occurs the fastest
reaction rate changes when optimum range isn’t maintained
✦﹒there are many factors that affect the enzyme—
how factors affect the enzyme activity in graphical form
pH levels
if outside optimum range → dec in enzyme activity, later denatured
temperature
inc in temp outside optimum range will inc activity, then later denature
dec in temp outside optimum range will slow activity, never denature
enzyme concentration
inc → inc activity
dec → dec activity
substrate concentration
inc will inc, and later stay constant to a certain point
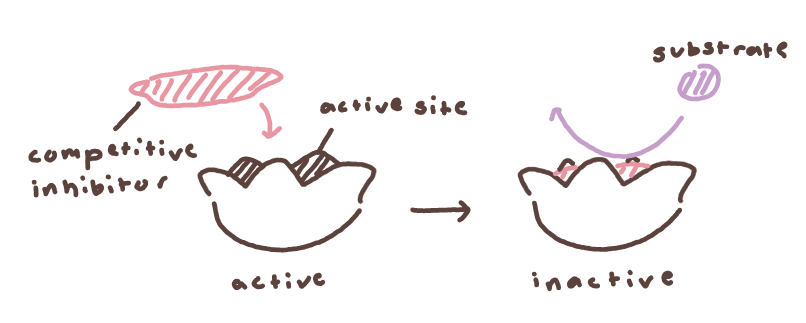
inhibitors, which can be either noncompetitive or competitive
noncompetitive - inhibitor binds to allosteric site, causing change in enzyme shape
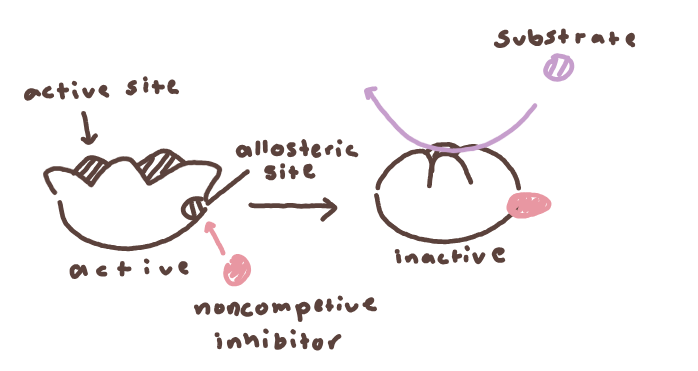
competitive - inhibitor competes with substrate, potentially alters reaction rate depending on amt
more inhibitors than substrate → slowed reaction
less inhibitors than substrate → normal reaction
can be reversible - enzyme is not affected by inhibitor binding
can be irreversible - enzyme is affected by inhibitor binding, permanently losing functionality via change in shape
✦﹒in a lab regarding enzymatic activity, there will be variables
positive control - confirms a known effect
negative control - confirms a result of absence in what is tested
control variable - aspects of experiment that are left unchanged, the baseline data
╭ Other Resources :
:: amoeba sisters ﹒﹒ goes over enzymes in general, briefly touches on factors that affect its activity near the end
﹙✦﹚﹒understanding the factors that affect enzyme reaction rate is key!!! def gonna be a frq/mcq asking about control variable
﹙3.4 - Cellular Energy﹚
✦﹒2nd law of thermodynamics states that ”entropy (disorder) must increase”
nature favors the process of going from order → disorder
exception is living things; they tend to go from disorder →order
every energy transfer increases disorder in the universe
✦﹒pathways in biological systems r sequential to allow for a more controlled and efficient transfer of energy
✦﹒metabolism is the sum of reaction in an organism
energy is constantly moving
catabolic reactions involve breaking down; lrg → small
also defined as exergonic
ex: cellular respiration, as sugars are broken down to ATP
another ex— ATP Hydrolysis (ATP + H2O→ ADP + Pi + free energy)
anabolic reactions involve building up; small → lrg
also defined as endergonic, as energy is used
ex: photosynthesis uses sun energy to build sugar from CO2,
another ex— ATP Synthesis (ADP + Pi + free energy → ATP + H2O)
﹙3.5 - Photosynthesis﹚
✦﹒photosynthesis is the biological process that captures light energy from the sun to produce energy
evidence supports the claim that prokaryotic photosynthesis by organisms, such as cyanobacteria, was responsible for the early production of O2 in the atmosphere
✦﹒light dependent reactions captures light energy by light absorbing molecules called pigments (chlorophyll)
helps transform light to chemical energy
light has electrons, powering NADP+ to NADPH
photosystem - light-capturing unit in a chloroplast’s thylakoid membrane
has photosystem I and II (PSI, PSII)
passes energy to electron transport chain
visual of light dependent reactions
✦﹒calvin cycle is the light independent reaction in which captured energy powers the production of carbs
uses ATP, NADPH + CO2 to make carbohydrates

﹙3.6 - Cellular Respiration﹚
process overview on cellular respiration
✦﹒electron transport chain (ETC) is a metabolic pathway tht efficiently transfers energy frm electrons to a proton gradient
electrons come frm electron carriers (NADH, FADH2)
active transport of proteins happen here
✦﹒oxidative phosphorylation is a process that uses ETCs, chemiosmosis, and ATP synthase to make ATP
NADH and FADH2 lose electrons to ETC (“oxidative”)
ATP synthase does ADP + Pi → ATP (“phosphorylation”)
in cellular respiration, the decoupling of this process frm the ETC generates heat
proton gradient is not being used by ATP synthase
heat can be used by endothermic organisms to regulate body temp

﹙3.7 - Fitness﹚
✦﹒fitness is the ability of the cell to survive and reproduce, often increased by variation
can provide organisms w/the ability to respond to a variety of environmental stimuli
can provide organisms w/fitness advantages under changing environment conditions
 Knowt
Knowt
