Mole: the unit of amount of substance in chemistry, its symbol is mol.
Stoichiometry: the relationship between the number of moles of reactant and the number of moles of products in the same chemical reaction.
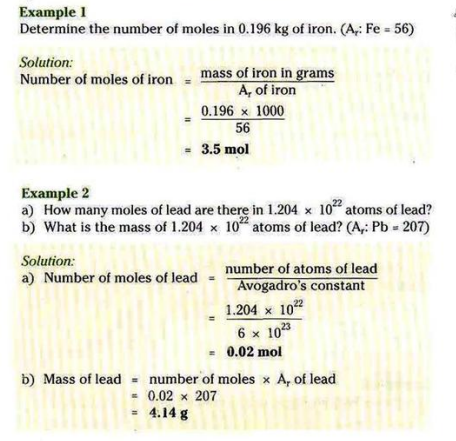
Relative Atomic Mass: the mass of one atom of an element compared to 1/12th of the mass of one Carbon-12 atom.
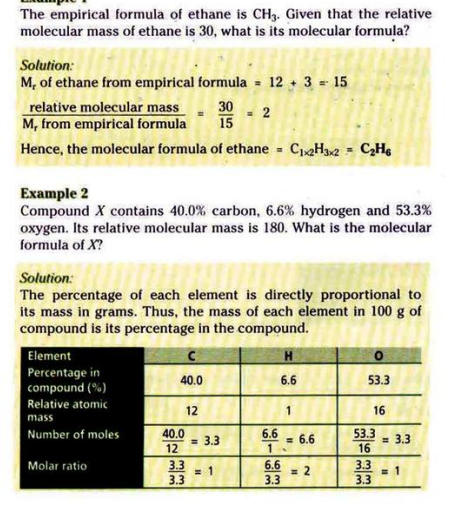
Relative Molecular Mass: the mass of one molecule of an element/compound compared to 1/12th of the mass of one Carbon-12 atom.
Relative Formula Mass: the molecular mass for ionic compounds.
Avagadro’s number/constant: one mole of any substance contains 6x1023 particles.
Empirical Formula: the simplest ratio of atoms or elements present in a compound.
Molecular Formula: shows the number of atoms of each element in a compound.
Molar Mass: Mass of 1 mole of a substance.
Molar Volume: Volume occupied by 1 mole of gas. At r.t.p, one mole of any gas occupies 24 dm3.
Concentration: Amount of solute dissolved per unit volume of the solution.
Yield: the amount of product formed in a reaction.
Theoretical yield: the calculated amount of product that would be obtained if the reaction would be complete with no external factors acting upon it.
Actual yield: the amount of product formed in a reaction..
Percentage yield: the relationship between the actual and theoretical yield.
Percentage purity: the percentage of the mass of pure substance upon mass of the whole sample.
Limiting reactant: the reactant that determines the amount of product formed because of having fewer moles.
Excess reactant: the leftover reactant after the reaction has stopped.
 Note
Note Studied by 8 people
Studied by 8 people Note
Note Studied by 105 people
Studied by 105 people Note
Note Studied by 1 person
Studied by 1 person Note
Note Studied by 62 people
Studied by 62 people Note
Note Studied by 19 people
Studied by 19 people Note
Note Studied by 23 people
Studied by 23 people Knowt
Knowt