Ionic and Covalent Bonds
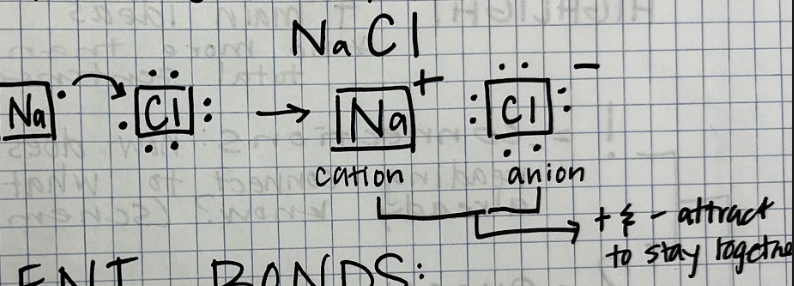
Ionic Bonds
Chemical bonds
Electrons are gained or lost
Occurs between metals and non-metals
+ = cation, - = anion
metal = cation
non-metal = anion
Example: Sodium Chloride
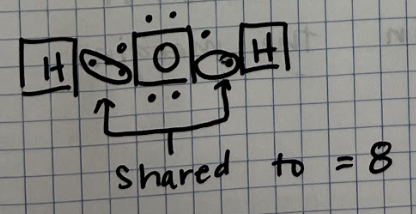
Covalent Bonds
Electrons are shared
Occurs between non-metals
Example: Water (H2O)