MECH 3542 Key Points 1
Lecture 1
Thermal expansion: 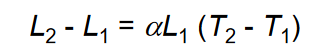 where alpha= coefficient of thermal expansion
where alpha= coefficient of thermal expansion
Thermal expansion is used to create shrink-fit assemblies, where the part is heated and cooled to expand or contract the geometry. Thermal expansion can be a concern in heat treatment and welding due to the thermal stresses that develop in the material.
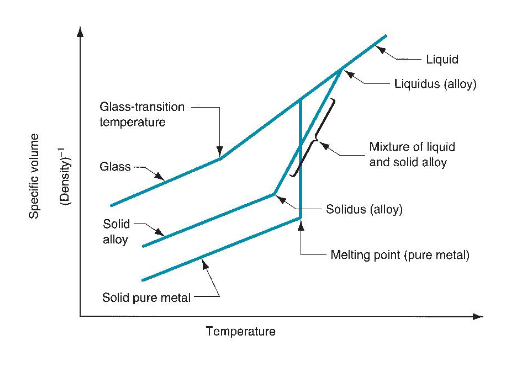
Melting point: temperature at which the material transforms from solid to liquid state
Heat of fusion: heat energy required at the melting point to transition from solid to liquid
For alloys, melting begins at the solidus temperature and continues until the liquidus temperature. Between the two temperatures, the alloy is a mix of solid and molten metals.
In noncrystalline materials (eg glass), the solid gradually softens as temperature increases and becomes a liquid at the melting point.
 Generally, thermal expansion properties for polymers are greater than metals. This is because metallic bonds are stronger than covalent bonds found in polymers.
Generally, thermal expansion properties for polymers are greater than metals. This is because metallic bonds are stronger than covalent bonds found in polymers.
Specific heat calculation: 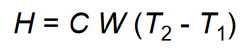 Volumetric specific heat = density * specific heat
Volumetric specific heat = density * specific heat
Thermal diffusivity calculation: 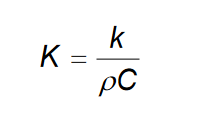 Thermal conductivity is typically higher for metals than ceramics or polymers, because metals have many free electrons
Thermal conductivity is typically higher for metals than ceramics or polymers, because metals have many free electrons
Copper is difficult to weld because of it’s high thermal conductivity.
In solids, electrons carry electric charge. In liquids, ions carry electric charge. Movement of charge is driven by voltage and resisted by inherent material characteristics.
Ohm’s law: V=IR
Resistivity calculation: 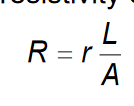 where r= resistivity, which defines the material’s capability to resist current flow. It is not constant, and varies with temperature. In metals, resistivity increased with temperature.
where r= resistivity, which defines the material’s capability to resist current flow. It is not constant, and varies with temperature. In metals, resistivity increased with temperature.
Conductivity=1/ r
Metals are the best conductors of electricity because of their metallic bonding. Conversely, ceramics and polymers, who have ionic or covalent bonds, are poor conductors.
Semiconductors: a material whose resistivity lies between insulators and conductors. Silicon is a common semiconductor.
Electrolyte: solution containing ions
Electrodes: where current enters and leaves the solution in the electrochemical process.
Anode: positive electrode that donates electrons to the other electrode
Cathode: negative electrode which gets plated
At each electrode, there is also a forming of gas. Positive ions in the electrolyte will be attracted to the negatively charged cathode. Negative ions will be attracted to the positively charged anode.