Chapter 12: Identification of Biological Evidence
12.1: Biological Properties
Blood constitutes about 8% of the human body weight of a healthy individual.
Plasma: The fluid portion of the blood.
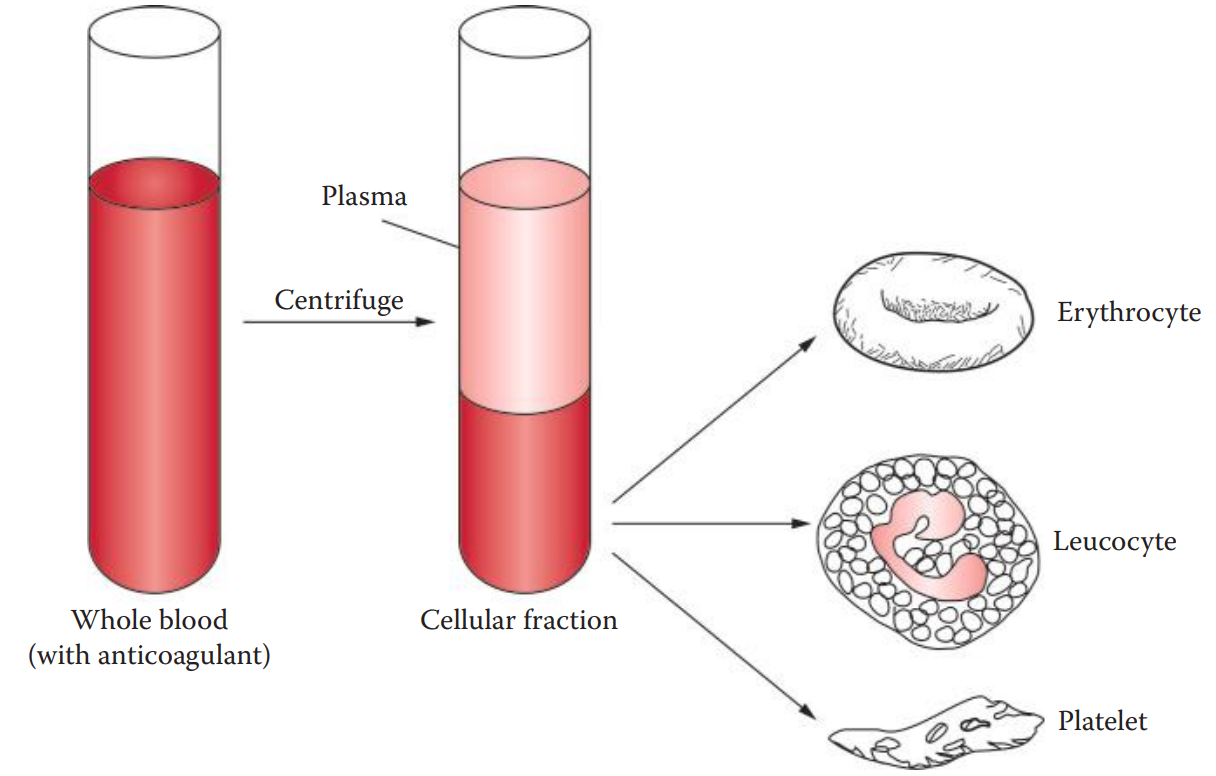
Red Blood Cells
- Also known as erythrocytes.
- Their life span in humans is approximately 3–4 months.
- They do not have nuclei, and therefore lack nuclear DNA.
- They consist of hemoglobin — proteins that are responsible for the transportation of oxygen.
- Ferroprotoporphyrin: A heme molecule.
White Blood Cells
- Also called leucocytes.
- They are involved in defending the body against infection.
- They have nuclei and thus represent the main sources of nuclear DNA from the blood.
Platelets
- Also known as thrombocytes.
- They play a role in blood clotting. They aggregate at sites of vascular and blood vessel injury.
- Like erythrocytes, they lack nuclei.
12.2: Presumptive Assays for Identification
Mechanisms of Presumptive Assays
- Presumptive blood assays are designed to detect traces of blood.
- These assays are based on the basic principle of the oxidation–reduction reaction catalyzed by the heme moiety of the hemoglobin.
- Oxidation–Reduction Reactions: These involve a change in the oxidation state of a molecule.
- The oxidation of a molecule means that the molecule has lost electrons, and the reduction of a molecule means that the molecule has gained electrons.
- Oxidants: Chemicals that can be reduced and gain electrons from other molecules.
- Reductants: Chemicals that can be oxidized and therefore lose electrons to other molecules.
Colorimetric Assays
Phenolphthalin Assay
Phenolphthalein: A member of a class of indicators and dyes, is used in titrations of mineral and organic acids as well as most alkalis.
The phenolphthalin assay for blood identification is also known as the Kastle–Meyer test.
It presents the results of a reaction in which phenolphthalin, a colorless compound, is catalyzed by heme with hydrogen peroxide as the oxidant.

Leucomalachite Green (LMG) Assay
Malachite green is a triphenylmethane dye.
The leuco base form of malachite green is colorless and can be oxidized by the catalysis of heme to produce a green color.
The reaction is carried out under acid conditions with hydrogen peroxide as the oxidant.
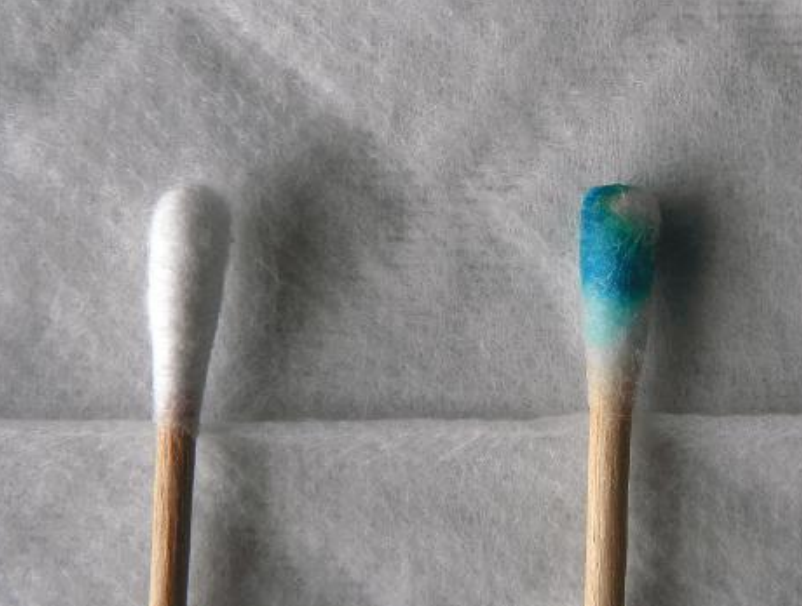
Benzidine and Derivatives
- Benzidine: Used a presumptive assay for the presence of blood after the discovery that the oxidation of benzidine can be catalyzed by heme to produce a blue to dark blue color.
- Orthotolidine: A dimethyl derivative of benzidine. Its oxidation reaction can be catalyzed by heme to produce a blue color reaction under acidic conditions
- Tetramethylbenzidine (TMB): A tetramethyl derivative of benzidine. Its oxidation can be catalyzed by heme to produce a green to blue-green color under acidic conditions.
Hemastix® assay kit: TMB-based assay that utilizes a TMB-containing strip device.
Chemiluminescence and Fluorescence Assays
In the chemiluminescence assay, light is emitted as a product of a chemical reaction.
Fluorescence Assay requires the exposure of an oxidized product, such as fluorescein, to a particular wavelength of an excitation light source.
Luminol
- It is usually utilized as a chemiluminescent reagent.
- The oxidation reaction of luminol catalyzed by heme produces light in the presence of an oxidant.
Fluorescein: It is used to test for the presence of bloodstains at a crime scene.

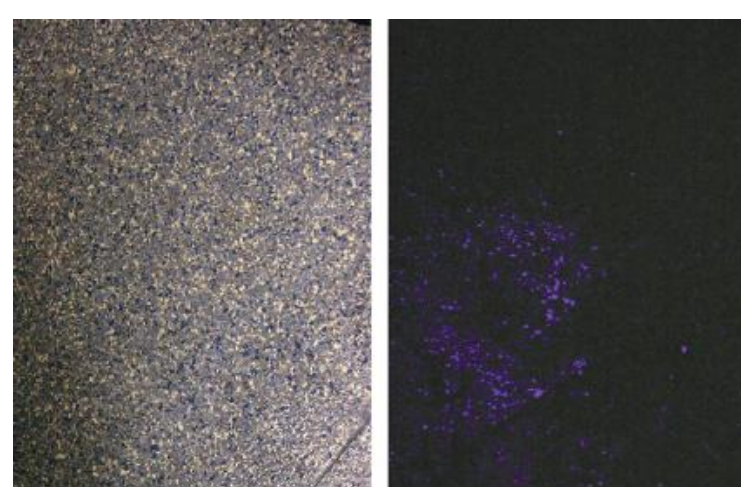
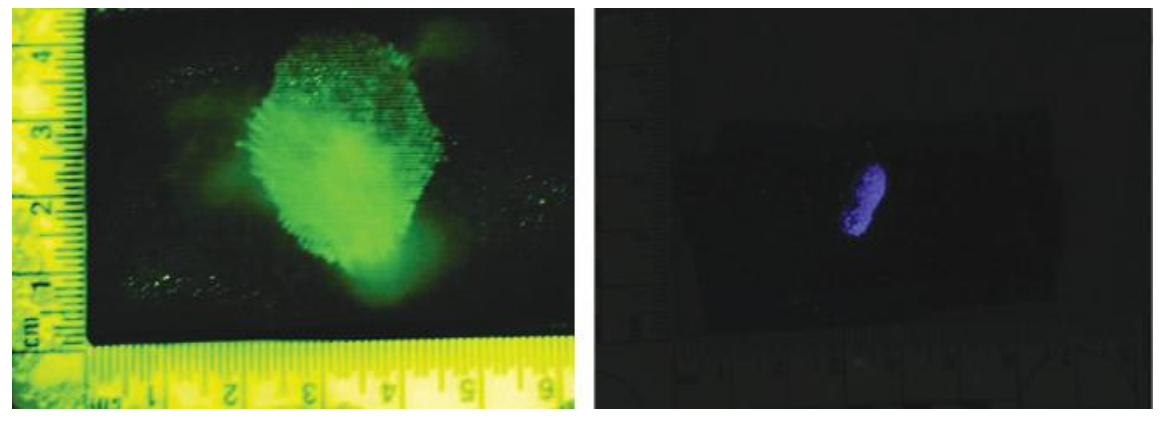
Factors Affecting Presumptive Assay Results
- Oxidants: Chemicals that are strong oxidants may cause a false-positive reaction.
- Plant Peroxidases: It may catalyze oxidation reactions and lead to false-positive results.
- Reductants: A false-negative result can occur when a strong reductant is present in a sample.
12.3: Confirmatory Assays for Identification
Microcrystal Assays
Microcrystal assays apply chemicals to treat bloodstains, forming crystals of heme molecules.
Hemochromagen Crystal Assay
- Hemochromagens are heme derivatives in which the ferrous iron of the heme forms two bonds with nitrogenous bases.
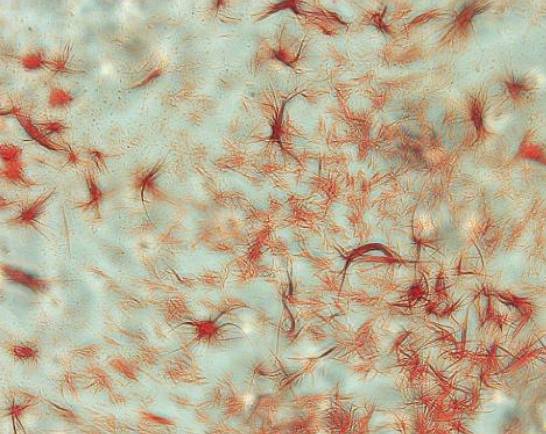
- Takayama Crystal Assay: A bloodstain is treated with pyridine and glucose under alkaline conditions to form crystals of pyridine ferroprotoporphyrin.
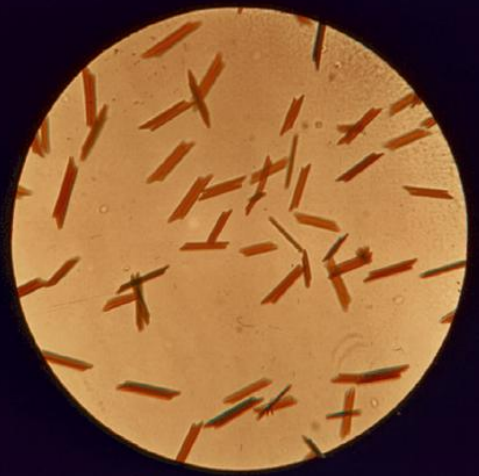
Hematin Crystal Assay
Also known as the Teichmann crystal assay.
When blood specimens are treated with glacial acetic acid and salts, and subsequently heated, hematin chloride a prismatic brown-colored crystal, is formed.
Hematin: A heme derivative; its iron is in the ferric state.
Other Assays
- Chromatographic and Electrophoretic Methods can identify hemoglobin by its mobility characteristics.
- Spectrophotometric methods for identifying hemoglobin are based on measurements of the characteristic light spectra, with a peak absorbance at 400–425 nm, absorbed by hemoglobin and its derivatives.
- Immunological methods utilize antihuman hemoglobin antibodies.
- This antibody can be used to detect human hemoglobin and thus indicate the presence of human blood.
- RNA-based assays: These assays are based on the fact that certain genes are specifically expressed in certain cell types.
- It is used in the identification of blood based on the detection of specific types of messenger RNA (mRNA) that are expressed exclusively in erythrocytes.