topic 6: energy and cellular metabolism
metabolism: sum of all chemical reactions in the body
anabolism: synthesis of organic molecules (endergonic reactions)
catabolism: breakdown of organic molecules(exergonic reactions)
Exergonic reactions release energy (spontaneous, negative ΔG), while endergonic reactions require energy input (non-spontaneous, positive ΔG)
control of chemical reactions
each reaction involves breaking bonds of reactants and forming new bonds in products
typically, free energy is released during the process
reactants have high energy state (low stability) and products have low energy state (high stability)
energy of chemical reactions measured in calories
1 calorie is amount of heat needed to increase temperature of 1 gram of water by 1 °C
what determines the rate of chemical reactions?
concentration of reactants - Law of mass action determines rate and direction of reaction
activation energy - the amount of energy needed by reactants to initiate breaking of chemical bond; involves collision of reactants with other molecules
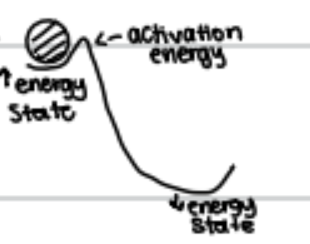
**the greater the amount of activation energy, the slower the reaction
temperature: increasing temeprature increases kinetic energy of molecules (rate of collision) thus increasing chance of acheiving activation energy
catalyst (enzyme) - acts to decrease energy of activation for reaction by altering charge distribution of reactants
**these reactions are reversible

**Maintaining structure of enzyme is critical to its function

binding and cataytic sites drift away when tertiery structure breaks down (by pH or temp)
increasing temp a little → sites become closer together → inc rate of rxn
increase temp too much → breaks down protein
Biochemistry (in body)
enzymes - proteins that increase reaction rate but do not cause the reaction to occur, they’re not used up during reaction
enzymes control activites and thus function of cells
enzymes increase rate of chemical reaction by lowering the activation energy
each cell contains ~4000 enzymes, dozens can be involved in a single metabolic pathway
substrates are the reactants in enzymatic reactions
substrate binding to active site of proteins, which has conplementary shape to substrate

formation of enzyme/substrate complez subject to same factors as receptor/ligand binding (affinity, specificity, competition, saturation)
activity of enzyme can also be regulated by molecules that are neither substrate nor product of that reaction
isozymes - same enzyme can exist in different states which alter the direction the direction of the same reaction
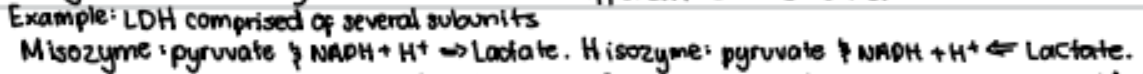
cofactors - some enzymes require presence of inorganic elements (trace elements like fe, mg, zinc)
cofactors do not directly participate in reaction, but cause conformational change in enzyme that increases binding of substrate
coenzymes - organic molecules (vitamins) tha directly participate in the rxn, but they are not used up
Factors Affecting Enzymatic Reactions
change in [substrate] : hormones play role in the (ec. catecholamines)
temperature
change in [enzymes]: involves transcriptional & translational regulation triggered by metabolic demand
in most reactions [substrate] > [enzymes]
in most cells [enzymes] determines role of reaction
changes in enzymatic activity: quicker method of altering rate of rxn then changing [enzyme]
how?
hormones act to phosphorylate (covalent modulation) enzymes. generally this increases rate of reaction
in metabolic pathways product of one reaction may alter enzyme structures of another reaction in the pathway
can increase or decrease activity (allosteric modulation)
atp is the common currency of all rxns in the body
Principle Functions of ATP
energize synthesis of improtant cellular components
energize muscle contractions
synthesis of organic molecules used for structure and function
energize active transport
keep cells “excitable”, Na+/K+ pump (in terms of muscle and neurons)
energy released from ATP by ATPase(enzyme)
ATP + water → ADP + Pi + 7,000 cals/mole
ATP stores 12,000 cals/mole, however real function of ATP is to transfer energy
energy stored in ATP (in the body) will sustain life for ~ 90 sec (without regeration)
phosphorylation (PC) is a major storage depot for energy (13,000 cals/mole)
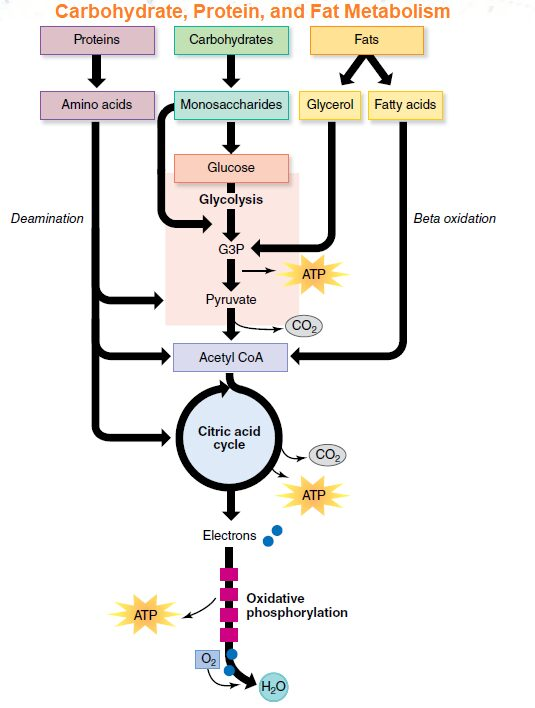
catabolic breakdown of food substrates leads to synthesis of ATP
carbs fats anf proteins
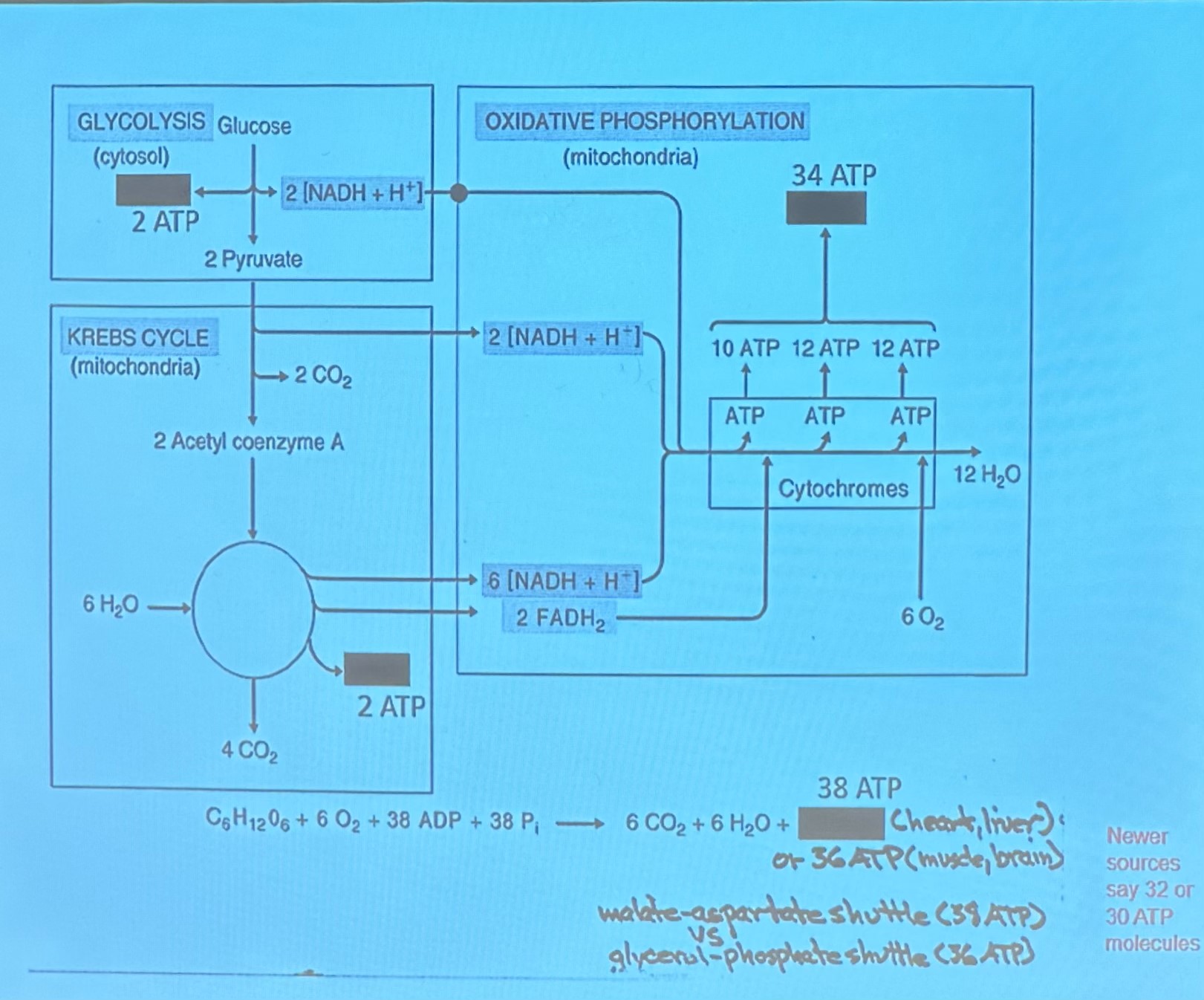
Breakdown of Sugar (Glycolsis) (blood sugar taken in and broken down)
consists of 10 step metabolic pathay, leading to synthesis of 2 pyruvate molecules and net production of 2 ATP molecules
all reactions occur in cytosol and non require oxygen
in step 5, the 6 carbon molecule cleaved into two 3 carbon molecules
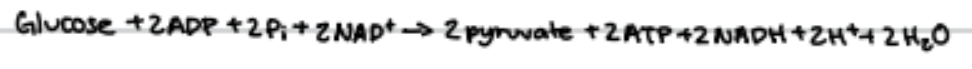
rate of pyruvate depends on avalability of oxygen
if inadequate oxygen avalable (anaerobic): pyruvate converted to lactate
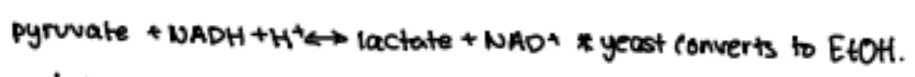
if enough oxygen available: pyruvate enter matrix of mitochondria and is converted to acetyl - CoA

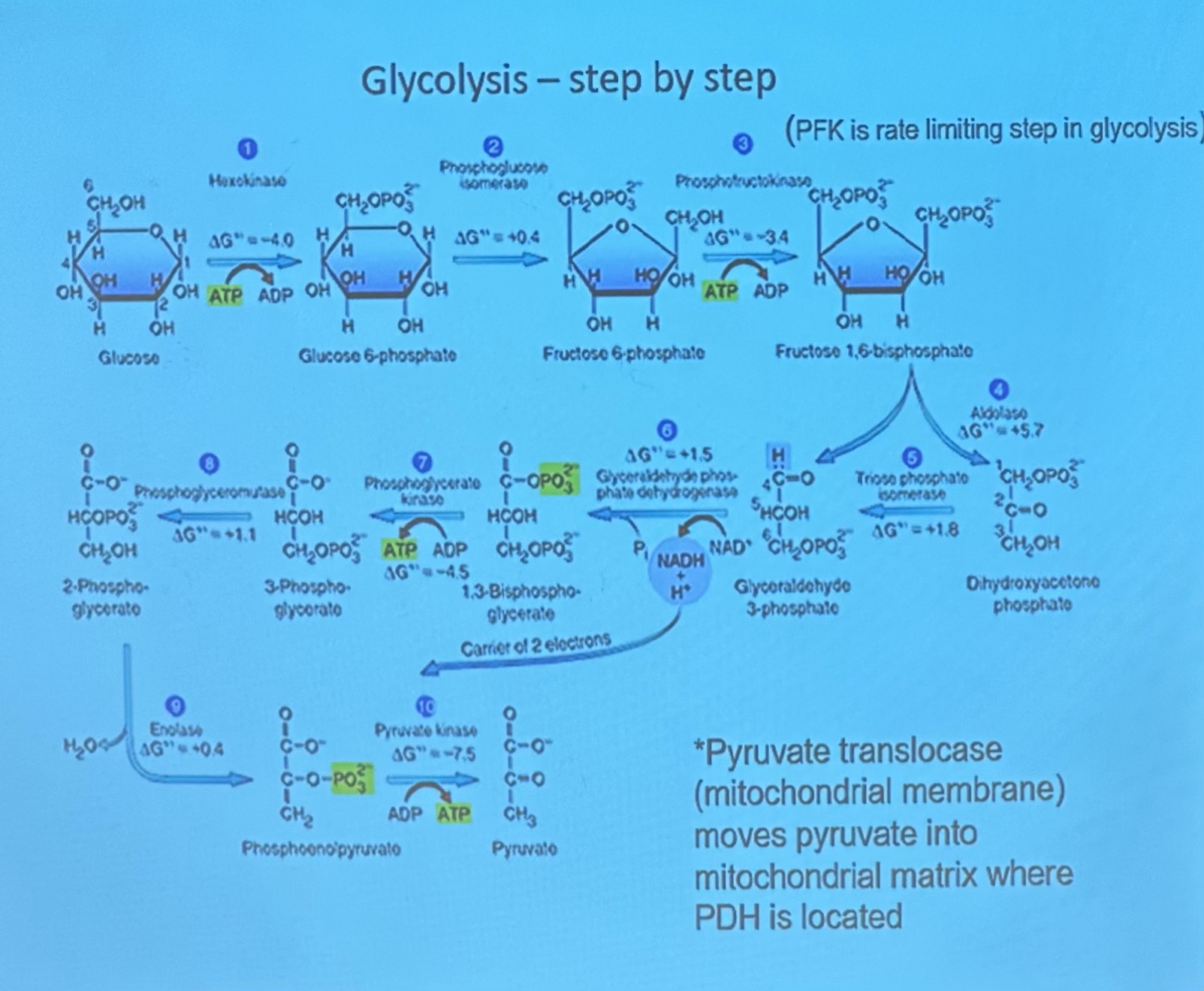
PFK IS THE LIMITING STEP IN GLYCOLSIS
Krebs Cycle is 8 step metabolic pathway
results in the production of
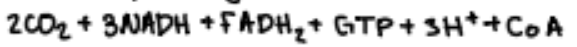
key is transfer of H to coenzymes (NAD+ + FAD), electrons can then be used in O/P to produce ATP
primary rate-limiting enzyme in the Krebs cycle, also known as the citric acid cycle or TCA cycle, is isocitrate dehydrogenase
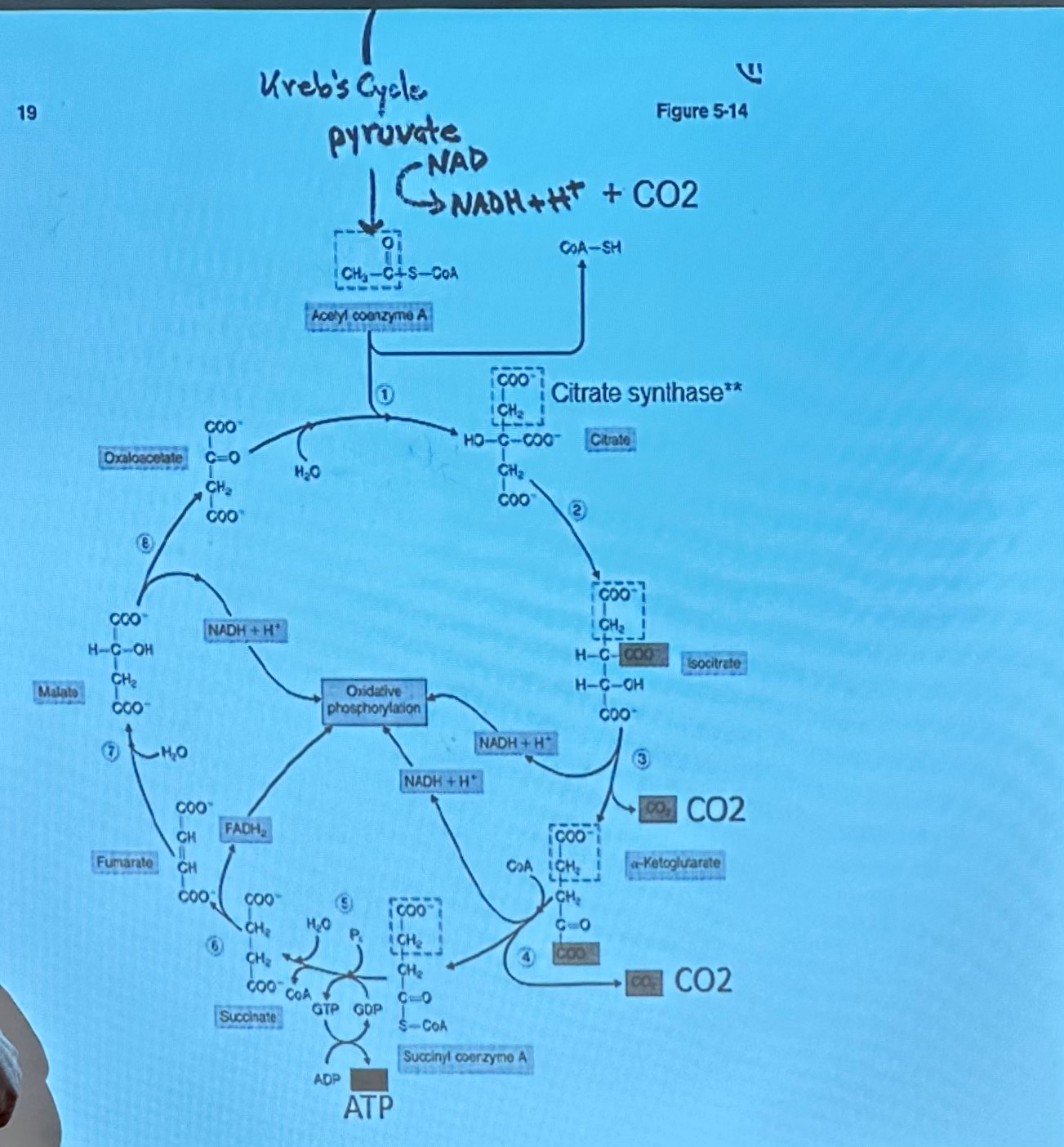
so at this point its 16 ATP but take away two so its 14 NET
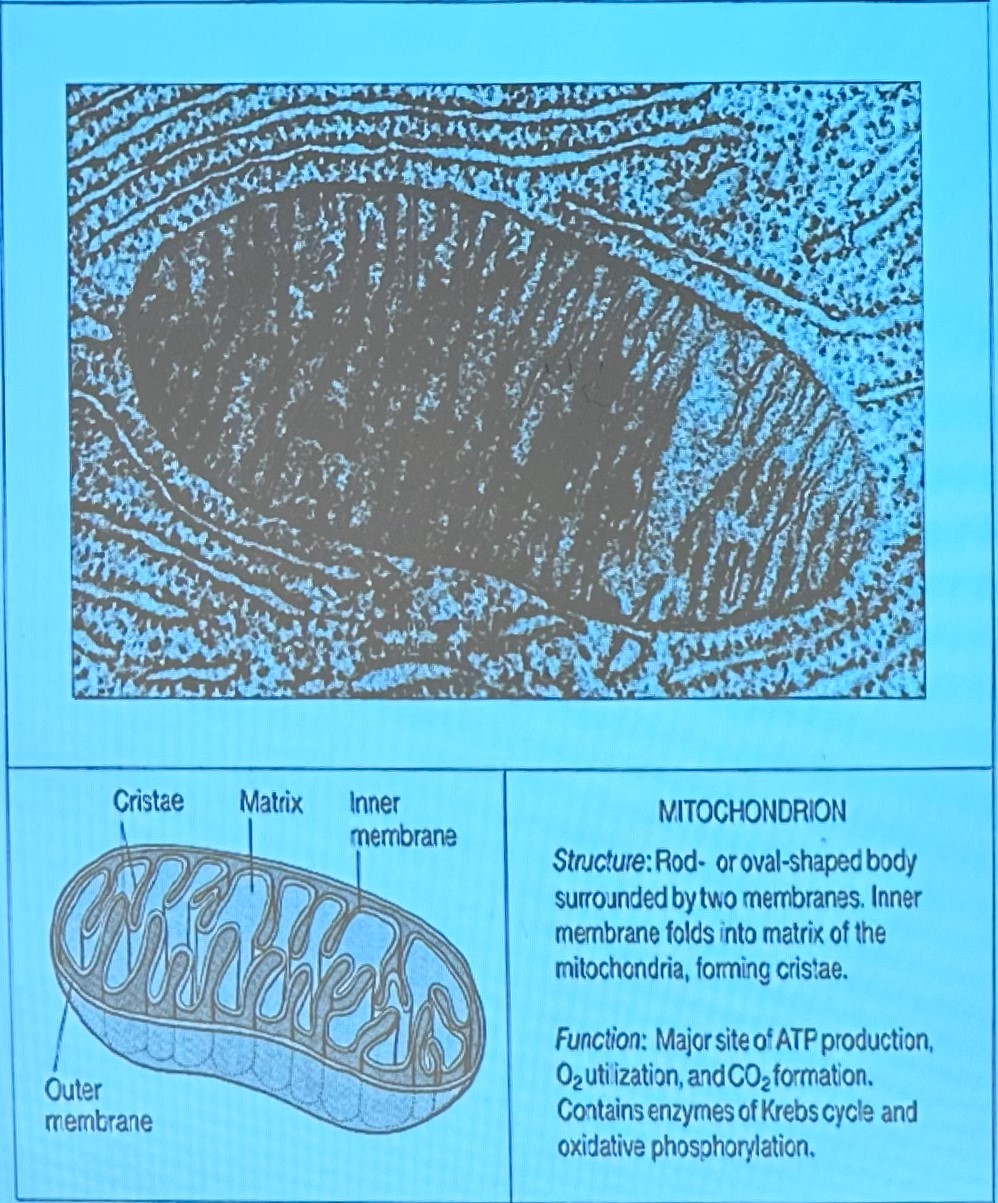
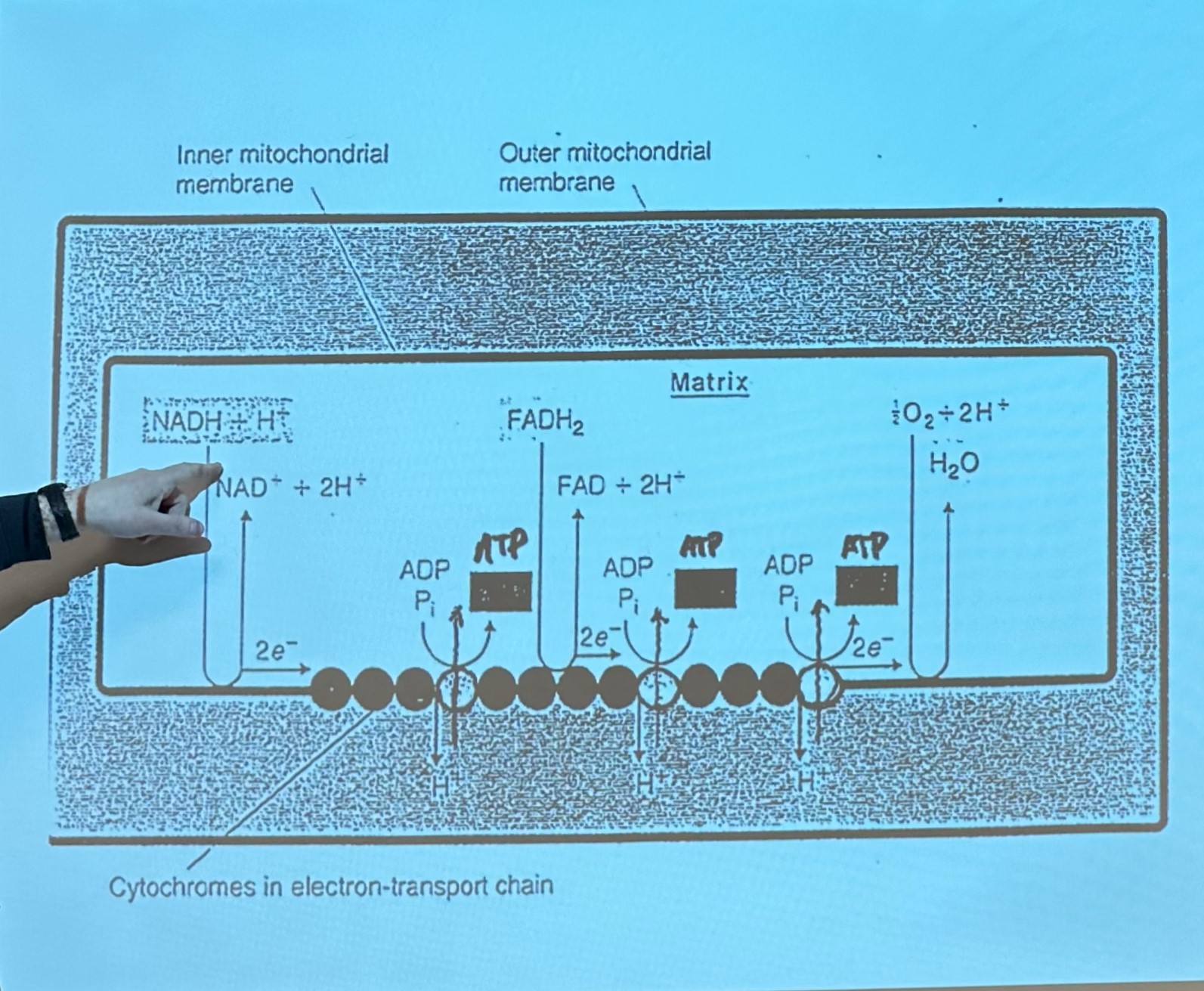
O/P occurs in inner membrane of mitochondria, results in transfer of electrons from NADH and FADH2 to oxygen to form H20
energy is released as electrons passed along cytochromes
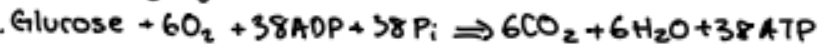
transfer electrons from cytosolic NADH into the mitochondria, allowing NADH to participate in oxidative phosphorylation (since NADH itself cannot cross the mitochondrial membrane)
Malate-Aspartate Shuttle (Heart, Liver, Slow-Twitch Muscle)
Glycerol-Phosphate Shuttle (Muscle, Brain, Fast-Twitch Fibers)
so efficient → only water as byproduct
electrons going down a hill (ETC)
higher energy to low energy states
Catabolic Breakdown of Fat (B - oxidation)
triacylglycerol composed of 3 fatty acids bound to glycerol backbone
accounts for 80% of stored energy in body
adipocytes synthesize and store triacylglycerols,
when energy is needed → release FAs and glycyerol into bloodstream
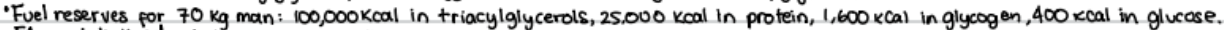
FAs metabolized only in the presence of oxygen
must first be activated by linking CoA to end of FA (requires energy)
activation occurs in cytoplasm, allows FA to be moves into mitochondrial matrix where CoA converted to acetyl-CoA
each round of B - oxidation cleaves off 2 carbon unit, and cleaves away the acteyl-CoA, and coennzymes reduced
acetyl-CoA enter Krebs cycle and is oxidized to 12 ATp molecules
FADH2 and NADH enter electron transport chain
FADH2 yields 2 ATP molecules
NADH yeilds 3 ATP molecules
each round of B - oxidation produces 17 ATP molecules
ex. Stearate is 18 carbon FA, B- oxidation produces:
9 acetyl coAs (108 ATP)
8 FADH2 (16 ATP)
8NADH (24 ATP)
total 148 produces but 2 are used to activate FA → net = 146 ATP
the first step in catabolizing fatty acids is activation
activation step only time when addition of CoA requires energy expenditure
B oxidation proceeds as follows
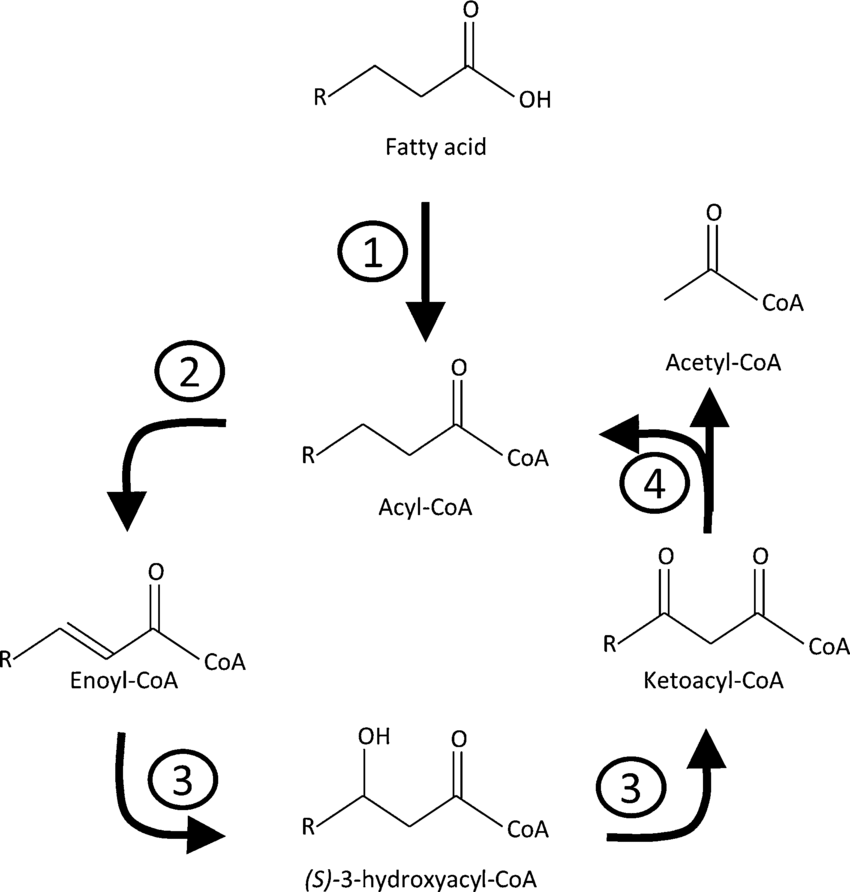
C - C - C - C - C - C - C - C - C - C 4 cuts
yields 5 acetyl COA units ( 5 × 12 ATPs ) = 60
yields 4 NADH units (4 × 3ATPS) = 12
yields 4 FADH2 units (4 × 2ATPs) = 8
total = 80 - 2(activation) net = 78
Catabolic Breakdown of Proteins
protesases cleave peptide bonds to free Amino acids
First step is to remove nitrogen atoms by either transamination or oxidase deamination
in oxidative deamination, the removed nitrogen found as ammonia (NH3)
ammonia converted to less toxic urea in liver and then excreted in urine
in transamination and oxidative deamination, AA’s are converted to keto acids which can be used as intermediates in krebs cycle or glycolysis to produce ATP
BCAA’s (branch chain AA) - can be used as energy substrates (enter TCA cycle) or for gluconeogenesis, or for muscle protein synthesis
isoleucine, leucine, valine