
Chapter 24
1. Define the following kidney-related terms: reabsorption, secretion, excretion, peristalsis, efflux, and reflux.
Reabsorption: This is the process by which substances are transported from the renal tubules back into the blood in the surrounding capillaries. Reabsorption allows the body to reclaim water, electrolytes, and essential nutrients from the glomerular filtrate to maintain homeostasis and prevent excessive loss of vital substances.
Secretion: This refers to the transport of substances from the blood into the tubular lumen. Secretion allows the kidneys to eliminate waste products and excess ions that are not required by the body, thus helping regulate body fluid composition.
Excretion: Excretion is the elimination of substances from the body, primarily through urine. It includes the removal of waste products, excess water, and solutes that are not reabsorbed during renal processes.
Peristalsis: Peristalsis is a series of wave-like muscle contractions that move contents through the digestive tract and other tubular structures in the body. In the context of the kidneys, peristalsis refers to the muscular movements in the ureters that propel urine from the renal pelvis to the urinary bladder.
Efflux: Efflux is the process of moving substances out of a compartment or structure. In the renal context, it typically refers to the movement of urine from the kidneys through the ureters to the bladder.
Reflux: Reflux is the backward flow of fluid, in this case, urine moving from the bladder back into the ureters or kidneys. This can occur due to anatomical defects or increased pressure within the bladder during micturition, which can lead to potential complications.
2. Review the structural and functional unit of the kidney.
The nephron is the structural and functional unit of the kidney, responsible for the formation of urine and the regulation of water, electrolytes, and waste products. Each nephron consists of several components:
Bowman Capsule: A cup-like sac that encases a tuft of capillaries (glomerulus). It is the site where filtration of blood begins, allowing water, ions, and small molecules to pass into the filtrate while retaining larger molecules like proteins.
Glomerulus: A network of capillaries within the Bowman capsule where blood filtration occurs. The glomerular capillaries are highly permeable and allow for the filtration of plasma while retaining cells and large proteins.
Proximal Convoluted Tubule (PCT): The segment following the Bowman capsule where significant reabsorption of water, ions (especially sodium), and nutrients (such as glucose and amino acids) occurs. This segment reabsorbs about 65-70% of the filtered sodium and water.
Loop of Henle: A U-shaped section consisting of a descending limb and an ascending limb. The descending limb is permeable to water but not to solutes, while the ascending limb is impermeable to water and actively transports sodium and chloride ions out, contributing to the concentration gradient in the medulla that aids in urine concentration.
Distal Convoluted Tubule (DCT): The segment that further modifies the filtrate. It reabsorbs sodium, chloride, and calcium, and is also involved in the secretion of potassium and hydrogen ions. The DCT is regulated by hormones such as aldosterone and parathyroid hormone.
Collecting Duct: The final segment of the nephron where the final concentration of urine occurs. The collecting duct reabsorbs water under the influence of antidiuretic hormone (ADH) and is responsible for the final composition and volume of urine that is excreted.
The nephron is surrounded by a rich blood supply that includes the afferent arterioles, which supply blood to the glomeruli, and the efferent arterioles, which carry blood away. The efferent arterioles further branch into the peritubular capillaries and vasa recta, which are involved in the reabsorption and secretion processes that take place along the nephron. Each nephron's design facilitates efficient filtration, selective reabsorption, and waste secretion, making the kidneys highly effective in maintaining homeostasis in body fluid composition.
3. List signs and symptoms of urinary tract disorders or diseases in children of different ages.
Neonatal Period (Birth to 1 Month)
Poor feeding
Vomiting
Failure to gain weight
Rapid respiration (often due to acidosis)
Respiratory distress
Spontaneous pneumothorax or pneumomediastinum
Frequent urination
Screaming on urination
Poor urinary stream
Jaundice
Seizures
Dehydration
Other anomalies or stigmata
Enlarged kidneys or bladder
Infancy (1 to 24 Months)
Poor feeding
Vomiting
Failure to gain weight
Excessive thirst
Frequent urination
Straining or screaming on urination
Foul-smelling urine
Pallor
Fever
Persistent diaper rash
Seizures (with or without fever)
Dehydration
Enlarged kidneys or bladder
Childhood (2 to 14 Years)
Poor appetite
Vomiting
Growth failure
Excessive thirst
Enuresis (bedwetting), incontinence, or frequent urination
Painful urination (dysuria)
Swelling of the face (periorbital edema)
Seizures
Pallor
Fatigue
Blood in urine (hematuria)
Abdominal or back pain
Edema (swelling)
Hypertension (high blood pressure)
Tetany (muscle cramps or spasms)
4. Identify nursing responsibilities in the assessment of renal disorders and diseases in children.
1. Ongoing Assessments
Weight and Height Monitoring: Accurate measurement and recording of the child’s weight and height are crucial to assess growth and fluid status.
Intake and Output (I&O): Careful tracking of fluid intake and urine output to identify potential imbalances or renal dysfunction.
Blood Pressure Measurement: Regular monitoring of blood pressure, as hypertension can be both a consequence and a contributing factor to renal disease.
2. Observation and Assessment
Clinical Manifestations: Observe for general and specific symptoms of renal dysfunction, such as changes in urine characteristics, signs of edema, pallor, or hypertension.
Symptom Recognition: Be vigilant for symptoms that may indicate complications or exacerbation of renal conditions, such as fever, vomiting, or abdominal pain.
3. Preparation for Diagnostic Tests
Patient and Family Education: Prepare infants, children, and their families for diagnostic tests and procedures, providing information about what to expect and how to prepare.
Urine and Blood Specimen Collection: Assist in the collection of urine and blood specimens, ensuring correct procedures are followed for accurate results.
4. Urine Analysis and Characteristics
Observation of Urine Characteristics: Monitor and record characteristics of urine, including color, clarity, odor, and volume.
Perform Urine Tests: Conduct tests on urine specimens, such as specific gravity, presence of protein, blood, glucose, and ketones.
5. Collaboration with Healthcare Team
Assist with Diagnostic Tests: Support and assist in more complex diagnostic tests, such as imaging studies or renal biopsies.
Familiarity with Laboratory Tests: Understand and interpret significant laboratory tests relevant to renal function and their implications for care.
6. Medication Safety and Alerts
Drug Alerts: Be aware of contraindications and risks associated with medications and interventions. For example, avoid using Fleet enemas in children with acute or chronic renal failure due to the risk of hyperphosphatemia and ensure thorough investigation before implementing such requests.
7. Monitoring for Complications
Identify At-Risk Patients: Recognize children at risk for renal complications (e.g., those in shock, postoperative patients) and prioritize assessments accordingly.
5. Outline the factors that contribute to the development of urinary tract infections (UTIs) in infants and children.
1. Microbial Factors
Common Uropathogens: Escherichia coli is the most prevalent cause of UTIs, especially in females. Other gram-negative bacteria associated with UTIs include Proteus mirabilis, Pseudomonas aeruginosa, Klebsiella, and Enterobacter. Gram-positive bacteria like Enterococcus and Staphylococcus saprophyticus can also cause UTIs, while viruses and fungi are rare causes in children.
Virulence Factors: These uropathogens possess specialized virulence factors that enable them to attach to and ascend the urinary tract. For instance, E. coli has pili and fimbriae that allow it to bind to the periurethral mucosa and move upward into the bladder.
2. Anatomical and Physiological Factors
Shorter Urethra in Females: Females have a shorter urethra, which provides an easier path for bacteria to reach the bladder. This anatomical feature, along with the closer proximity to the gastrointestinal tract, increases the risk of bacterial migration.
Uncircumcised Males: The presence of a foreskin in males is associated with a higher risk of UTI due to increased periurethral bacterial colonization. Studies show that uncircumcised infants have a significantly higher incidence of UTI compared to circumcised males.
Urinary Stasis: The most significant host factor for UTI development is urinary stasis. When the bladder is not completely emptied, it allows bacteria to persist and multiply. Causes of urinary stasis include:
Anatomic Abnormalities: Conditions like vesicoureteral reflux (VUR), where urine flows backward from the bladder to the ureters, contribute to urinary retention.
Functional Issues: Conditions like neurogenic bladder and dysfunctional voiding lead to incomplete bladder emptying, increasing UTI risk.
3. Functional and Behavioral Factors
Bowel-Bladder Dysfunction (BBD): BBD is characterized by symptoms like urinary frequency, urgency, and constipation, all of which increase UTI risk by promoting urinary stasis. It often occurs in children with pelvic floor dysfunction or those with abnormal voiding habits developed during or after toilet training.
Holding Back Urine: Children may voluntarily hold back urine, leading to bladder overdistention and incomplete emptying. This creates an environment favorable for bacterial growth.
Dysfunctional Voiding: Some children contract the urethral sphincter while urinating, leading to urinary stasis and increased risk of infection.
4. Extrinsic Factors
Constipation: A full rectum can press against the bladder, causing incomplete emptying and urinary stasis. Treating constipation is essential for reducing UTI recurrence.
Catheter Use: Indwelling catheters can introduce bacteria into the urinary tract, increasing infection risk, particularly with prolonged use.
Antibiotic Use: Antibiotics disrupt normal perineal flora, which can make it easier for pathogenic bacteria to colonize the urinary tract.
5. Environmental and Hygienic Factors
Poor Hygiene: Improper cleaning practices, especially in young children, can introduce bacteria into the urethra, increasing the risk of infection.
Irritants: Bubble baths, essential oils, and some shampoos can irritate the urethra, causing frequent and painful urination.
Hot Tubs and Whirlpools: The use of hot tubs or whirlpool baths is associated with an increased risk of UTIs due to bacterial exposure.
Sexual Activity (in Older Children): Sexual intercourse may introduce bacteria into the urinary tract, leading to transient bacteriuria and increased UTI risk in females.
6. Define the following terms used to classify UTIs and urinary inflammation: bacteriuria, asymptomatic bacteriuria, symptomatic bacteriuria, recurrent UTI, persistent UTI, febrile UTI, cystitis, urethritis, pyelonephritis, and urosepsis.
Bacteriuria: Presence of bacteria in the urine.
Asymptomatic Bacteriuria: Significant bacteriuria (typically >100,000 colony-forming units per mm³) without clinical symptoms of infection.
Symptomatic Bacteriuria: Bacteriuria accompanied by symptoms of a urinary infection, such as dysuria (painful urination), suprapubic discomfort, hematuria (blood in urine), or fever.
Recurrent Urinary Tract Infection (UTI): Repeated episodes of bacteriuria or symptomatic UTI.
Persistent UTI: Continued presence of bacteriuria despite appropriate antibiotic treatment.
Febrile UTI: Bacteriuria with accompanying fever and other physical signs of infection; fever often suggests that the infection has reached the kidneys (pyelonephritis).
Cystitis: Inflammation of the bladder.
Urethritis: Inflammation of the urethra.
Pyelonephritis: Inflammation of the upper urinary tract, particularly affecting the kidneys.
Urosepsis: A febrile UTI associated with systemic signs of infection, often confirmed by the presence of urinary pathogens in the bloodstream (positive blood culture).
7. Discuss the clinical manifestations of UTIs in children of various ages.
Infants and Toddlers (under 2 years old):
Often present with nonspecific symptoms such as fever, irritability, lethargy, poor feeding, vomiting, and diarrhea.
Newborns may show fever or hypothermia, jaundice, tachypnea, or cyanosis and may appear significantly ill.
Children Over 2 Years:
More likely to exhibit classic UTI symptoms, including enuresis (bedwetting) or daytime incontinence in previously toilet-trained children, fever, foul-smelling urine, increased urination frequency, dysuria (painful urination), and urgency.
Some may report abdominal pain or flank pain (costovertebral angle tenderness).
Additional symptoms may include hematuria (blood in urine) or vomiting.
In younger boys, obstructive symptoms like dribbling of urine, straining, or reduced urine stream force may appear.
Symptoms of Pyelonephritis:
High fever, chills, and flank pain may suggest kidney infection.
Severe abdominal pain and leukocytosis (high white blood cell count) can also indicate pyelonephritis.
Physical examination may reveal flank tenderness as the primary sign.
Older Children and Adolescents:
Lower urinary tract infections (bladder or urethra) generally cause frequency, urgency, and painful urination. The urine may be cloudy or blood-tinged, and fever is either absent or low-grade.
Upper urinary tract infections (kidney involvement) are usually marked by higher fever (>38°C), chills, and flank pain, sometimes combined with symptoms of lower tract infections.
Asymptomatic or Atypical Presentations:
Many children have asymptomatic or atypical presentations, with symptoms that may resemble respiratory or gastrointestinal infections.
Identifying UTIs in these cases is critical to prevent renal scarring, especially in infants and young children.
Dysfunctional Voiding Symptoms :
Includes urinary frequency changes, constipation, straining, urgency, squatting to prevent wetting, and daytime/nighttime wetting.
8. Describe common side effects of urinary antiinfective agents and nursing interventions to minimize these side effects.
DrugSide EffectsNursing Interventions | ||
Trimethoprim-sulfamethoxazole (Bactrim, Septra) | Rash, urticaria, photosensitivity, nausea, bone marrow depression (long-term use) | - Encourage adequate fluid intake to prevent crystalluria. |
Amoxicillin (Amoxil, Polymox, Trimox) | Nausea, vomiting, diarrhea | - Refrigerate the suspension and discard after 14 days. |
Nitrofurantoin (Macrodantin, Furadantin) | Nausea, pneumonitis, or pulmonary fibrosis (long-term use) | - Administer with food or milk to decrease nausea. |
Cephalexin (Keflex) | Nausea, diarrhea | - Administer with food or milk. |
Ceftazidime (Fortaz) | Renal toxicity | - Monitor renal function through lab values, such as BUN and creatinine. |
Gentamicin (Garamycin) | Renal toxicity, ototoxicity | - Monitor renal function closely. |
Additional Care and Interventions for Suspected Pyelonephritis:
Hospital Admission: Infants and young children with suspected pyelonephritis and fever may require hospital admission for intravenous (IV) antibiotics and hydration.
Culture Testing: Blood and urine cultures are typically obtained upon admission and after therapy to monitor infection response.
Nitrofurantoin Use Limitation: Nitrofurantoin is avoided in febrile infants or in cases of suspected pyelonephritis, as it does not reach therapeutic levels in the blood or kidney.
9. Discuss vesicoureteral reflux (VUR) and the International Classification System used to describe the level of reflux into upper urinary tract structures.
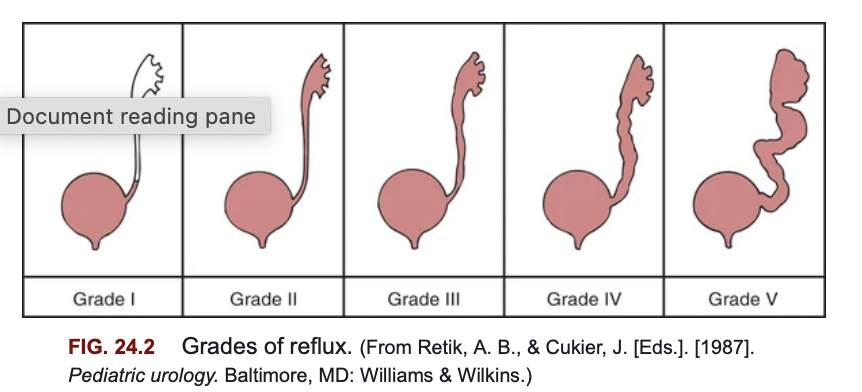
Vesicoureteral reflux (VUR) involves the backward flow of urine from the bladder into the ureters and sometimes up to the kidneys. While VUR itself does not directly cause urinary tract infections (UTIs), it increases the risk of febrile UTIs and may contribute to kidney damage if the refluxing urine carries bacteria to the upper urinary tract. The pressure during voiding or filling of the bladder can force urine back into the ureters, filling the renal pelvis and potentially leaving residual urine in the ureter after urination, which increases infection risk.
International Classification System for VUR
The International Classification System categorizes VUR into five grades based on the degree to which urine refluxes into the upper urinary tract structures:
Grade I: Urine refluxes only into the ureter, without reaching the renal pelvis.
Grade II: Reflux reaches the renal pelvis but without any dilation of the ureter or renal pelvis.
Grade III: Reflux reaches the renal pelvis, causing mild to moderate dilation of the ureter and renal pelvis.
Grade IV: Reflux causes moderate dilation of the ureter and renal pelvis with mild blunting of the renal calyces.
Grade V: Severe dilation of the ureter, renal pelvis, and calyces, along with loss of the normal papillary impressions, which suggests more severe kidney involvement.
Types and Causes of VUR
Primary VUR: Caused by a congenital defect at the ureterovesical junction, where the ureter enters the bladder. In this case, the anti-reflux mechanism fails, allowing urine to flow backward.
Secondary VUR: Occurs due to abnormally high bladder pressure from anatomical (e.g., posterior urethral valves) or functional causes (e.g., neurogenic bladder or dysfunctional voiding). Children with neurogenic bladder conditions, such as spina bifida, are particularly susceptible to secondary VUR.
10. Discuss the therapeutic management and nursing care indications for children with VUR.
Therapeutic Management of VUR
Nonoperative Therapy:
Most cases of vesicoureteral reflux (VUR) resolve spontaneously over time, especially in younger children, lower grades of reflux, unilateral reflux, and those diagnosed early (under 1 year). Higher grades of VUR (IV and V) have lower spontaneous resolution rates.
Continuous Antibiotic Prophylaxis (CAP): Used to prevent bacterial multiplication in the urine, CAP involves giving antibiotics such as trimethoprim-sulfamethoxazole, trimethoprim, or nitrofurantoin, usually at bedtime. Amoxicillin may be used in infants under 2 months but is avoided in older children to reduce antibiotic resistance.
CAP is usually continued until VUR resolves. However, treatment is increasingly individualized, considering factors like the child’s toilet training, voiding habits, and family preferences. Parents and caregivers must closely monitor the child for any signs of UTI or unexplained fever, as breakthrough infections can still occur despite CAP.
Surgical Management:
Surgical intervention may be necessary in cases where reflux is unlikely to resolve and poses a risk of renal damage (e.g., Grade V with renal scarring, high-grade VUR in children older than 6, or recurrent UTIs despite CAP).
Surgical Options:
Open Surgical Correction: Involves reimplanting the ureter to correct the abnormal insertion into the bladder. This procedure requires a short hospital stay.
Endoscopic Correction: A minimally invasive procedure that injects a bulking agent (dextranomer/hyaluronic acid) beneath the ureterovesical junction mucosa to alter the ureter’s angle and reduce reflux. This outpatient procedure is less invasive but may be less durable over time, with some children experiencing recurrence of reflux.
Postoperative Monitoring:
Renal ultrasonography is performed one month after surgery to monitor for ureteral obstruction and confirm surgical success.
Nursing Care Management for Children with VUR
Promoting Compliance with Medical Therapy:
Educate parents and older children on the importance of adhering to the prescribed antibiotic regimen to prevent UTIs.
Provide guidance on methods to encourage medication compliance, especially in young children, and emphasize hygiene practices and frequent voiding schedules.
Inform parents about recognizing UTI symptoms and the need for prompt medical attention, as breakthrough infections can occur even with CAP.
Sibling Screening and Family Education:
Since VUR has a familial tendency, nurses should inform parents of the potential risk for siblings. Screening with renal ultrasound or cystography is recommended if there is a family history of UTIs or abnormal findings on ultrasound.
Encourage awareness of VUR symptoms in other children and prompt evaluation if there are signs of UTI.
Preparation and Support for Procedures:
Prepare children appropriately for diagnostic procedures (e.g., renal ultrasound, VCUG) and consider using lidocaine jelly to ease discomfort during catheterization.
Ensure that children and parents understand the process and are comfortable with it, as preparation can reduce anxiety and improve cooperation.
Follow-Up and Support:
Follow-up care is essential, especially in children with high-grade VUR or frequent UTIs. Regular monitoring of renal health and growth is crucial to prevent long-term complications such as renal scarring or chronic kidney disease.
11. Identify the etiology and common signs and symptoms of acute glomerulonephritis (AGN) as a primary event and as a manifestation of a systemic disorder.
Etiology:
Primary Event: AGN can develop primarily within the kidney due to an immune response or infection, often as a postinfectious reaction to certain bacteria (e.g., group A β-hemolytic streptococcus) or viruses. The infection triggers immune complex formation, which deposits in the glomeruli and leads to inflammation.
Systemic Disorder: AGN can also manifest as part of a systemic condition, such as lupus nephritis or Henoch-Schönlein purpura, where the body’s immune response affects multiple organs, including the kidneys.
Signs and Symptoms:
Oliguria (reduced urine output)
Edema, especially facial/periorbital, spreading to extremities and abdomen
Hypertension due to fluid retention and circulatory congestion
Hematuria (cola or tea-colored urine) indicating blood in the urine
Proteinuria (presence of protein in the urine)
Other general symptoms: irritability, lethargy, anorexia, headache, abdominal discomfort, and in severe cases, dysuria.
12. Discuss the role of poststreptococcal infection in the incidence of acute poststreptococcal glomerulonephritis (APSGN).
Role of Poststreptococcal Infection in Acute Poststreptococcal Glomerulonephritis (APSGN)
APSGN is the most common noninfectious renal disease in children and typically follows a latent period of 1-2 weeks after a streptococcal throat infection or 3-6 weeks after a skin infection like impetigo.
APSGN occurs due to an immune-complex reaction involving antibodies formed in response to streptococcal antigens. These immune complexes become trapped in the glomeruli, causing inflammation and structural changes.
APSGN often appears in early school-age children (3-12 years) and is most prevalent following streptococcal infections in colder months or during late summer and early fall in warmer climates.
13. Describe the major complications of AGN.
Major Complications of AGN
Hypertensive Encephalopathy:
Cause: Severe, acute hypertension can lead to a failure of cerebral autoregulation, causing brain hyperperfusion and cerebral edema.
Signs: Headache, dizziness, vomiting, blurred vision, disorientation, and seizures.
Acute Cardiac Decompensation:
Cause: Hypervolemia, rather than heart failure, increases fluid load on the heart, causing circulatory congestion and potentially pulmonary edema.
Signs: Enlarged heart, increased pulmonary vascular markings, and possibly respiratory symptoms.
Acute Kidney Injury (AKI):
Cause: Severe and prolonged glomerular inflammation may lead to oliguria or anuria.
Signs: Persistent low urine output, increased blood urea nitrogen (BUN) and creatinine, potentially necessitating supportive therapy.
14. Discuss the pathophysiology, clinical manifestations, and therapeutic management for a child with chronic glomerulonephritis.
Pathophysiology of Chronic Glomerulonephritis (CGN)
Chronic glomerulonephritis (CGN) encompasses a group of kidney disorders characterized by long-term inflammation and damage to the glomeruli, which are the filtering units of the kidney. The pathophysiological processes involved in CGN can be multifactorial and include:
Immunologic Mechanisms: CGN is often associated with immune-mediated injury. This may occur through:
Direct Attack: Autoantibodies target kidney tissues, leading to inflammation and damage.
Immune Complex Deposition: Immune complexes formed by antibodies and antigens can deposit in the glomeruli, activating the complement system and causing inflammation.
Secondary Immune Response: Previous kidney injury may result in chronic inflammation and fibrosis as the immune system attempts to repair the damage.
Structural Changes: The chronic inflammation leads to glomerular scarring (sclerosis) and damage, ultimately affecting kidney function. Over time, the glomeruli may lose their ability to filter blood effectively, resulting in nephron loss and renal fibrosis.
Progression to End-Stage Renal Disease (ESRD): The long-term consequences of CGN include progressive renal insufficiency. As nephron damage accumulates, renal function declines, leading to the potential need for dialysis or kidney transplantation.
Clinical Manifestations
The clinical presentation of CGN can vary widely, especially depending on the underlying cause. Early in the disease, symptoms may be minimal or asymptomatic. However, as the disease progresses, the following manifestations may occur:
Proteinuria: This can range from mild to nephrotic syndrome levels, often detectable on routine urinalysis.
Hematuria: Microscopic or gross hematuria may be present.
Hypertension: High blood pressure is common due to fluid overload and renal impairment.
Edema: Swelling may occur, particularly in the face, abdomen, or legs, due to fluid retention.
Signs of Chronic Kidney Disease: These may include fatigue, anemia, and electrolyte imbalances (e.g., hyperkalemia, metabolic acidosis).
Growth Retardation: In children, chronic kidney disease can impact growth and development.
Therapeutic Management
Management of CGN aims to address the underlying cause, control symptoms, and preserve kidney function. Key components include:
Symptomatic Treatment: Early stages may require symptomatic management, focusing on optimizing the child’s health, growth, and quality of life. This includes:
Dietary modifications: Reduced sodium and protein intake may be recommended to manage edema and renal workload.
Fluid management: Careful monitoring of fluid intake to prevent overload.
Pharmacological Interventions:
Corticosteroids: Used in some cases, particularly those associated with nephrotic syndrome or significant inflammation.
Immunosuppressive agents: Cytotoxic medications may be indicated in specific types of CGN.
Antihypertensive Agents: ACE inhibitors or angiotensin II receptor blockers (ARBs) to manage hypertension and protect kidney function.
Erythropoietin: Administered for anemia management in patients with chronic kidney disease.
Monitoring and Follow-Up: Regular monitoring of renal function, electrolytes, and overall health is crucial.
Renal Replacement Therapy: In advanced stages, when renal function is severely compromised, dialysis or kidney transplantation may be necessary.
Nursing Care Management
Nursing care for children with CGN includes:
Education and Support: Providing information about the disease process, treatment options, and the importance of adherence to therapy.
Monitoring: Regular assessment of vital signs, weight, and laboratory values to detect complications early.
Psychosocial Support: Addressing the emotional and developmental needs of the child, encouraging normalcy in daily activities as much as possible.
Coordination of Care: Collaborating with a multidisciplinary team to provide comprehensive care tailored to the child’s needs.
15. Name the three types of nephrotic syndrome and common clinical manifestations, therapeutic management, and nursing care management for children with these conditions.
Nephrotic syndrome in children can be classified into three main types: Minimal Change Nephrotic Syndrome (MCNS), Secondary Nephrotic Syndrome, and Congenital Nephrotic Syndrome (Finnish Type). Below is an overview of each type, common clinical manifestations, therapeutic management, and nursing care management associated with these conditions.
1. Minimal Change Nephrotic Syndrome (MCNS)
Common Clinical Manifestations:
Rapid weight gain due to edema, particularly around the eyes in the morning and progressing to abdominal and lower extremity swelling.
Generalized edema (anasarca) can develop.
Decreased urine output, often dark and frothy.
Possible abdominal pain and diarrhea from intestinal edema.
Pallor, fatigue, and irritability.
Susceptibility to infections, particularly cellulitis and pneumonia.
Skin changes, such as white lines (Muehrcke lines) on nails due to hypoalbuminemia.
Therapeutic Management:
Corticosteroid Therapy: Prednisone is the first-line treatment, with a typical dosage of 60 mg/m²/day for 4-6 weeks, then tapered.
Diuretics: May be used to manage edema, though their effectiveness can be limited.
Supportive Care: Treatment of infections, nutritional support, and monitoring of fluid status.
Immunosuppressive Therapy: For steroid-resistant or frequently relapsing cases, medications like cyclophosphamide or rituximab may be considered.
Nursing Care Management:
Daily monitoring of weight, intake/output, and abdominal girth.
Assessment of edema and skin integrity.
Infection prevention strategies, including hand hygiene and isolation from sick individuals.
Nutritional guidance, including managing dietary sodium and providing small, frequent meals.
Education for parents on recognizing signs of relapse and administering medications.
2. Secondary Nephrotic Syndrome
Common Clinical Manifestations:
Similar to MCNS but with an underlying cause, such as infections, drugs, or systemic diseases (e.g., lupus).
The clinical presentation can vary depending on the associated condition.
Therapeutic Management:
Treat Underlying Cause: Addressing the primary condition (e.g., treating infections, adjusting medications).
Supportive Care: Similar to MCNS, including corticosteroids if indicated.
Nursing Care Management:
Close monitoring for signs of complications related to the underlying disease.
Family education regarding the systemic illness and its management.
Coordination of care across specialties as needed.
3. Congenital Nephrotic Syndrome (Finnish Type)
Common Clinical Manifestations:
Symptoms manifest within the first few days to months of life, including significant proteinuria and edema.
Infants are typically small for gestational age.
Severe and progressive course, often not responsive to standard therapies.
Therapeutic Management:
Supportive Care: IV albumin, nutritional support, dialysis, or kidney transplant may be necessary.
Close monitoring of growth and metabolic status.
Nursing Care Management:
Intensive monitoring of weight and nutritional status.
Education and support for families regarding the prognosis and care needs.
Emotional support for families facing the challenges of congenital conditions.
General Considerations for All Types
Monitoring and Assessment: Daily measurements of vital signs, weight, and fluid status are crucial.
Infection Control: High susceptibility to infections necessitates protective measures.
Family Support: Providing education and emotional support to families is critical, especially during hospitalizations or periods of frequent relapses.
16. Discuss the clinical manifestations and management of the following renal diseases: nephrogenic diabetes insipidus, hemolytic uremic syndrome, familial nephritis (Alport syndrome), and unexplained proteinuria.
Nephrogenic Diabetes Insipidus (NDI)
Clinical Manifestations: NDI is characterized by the kidneys' inability to concentrate urine due to insensitivity to antidiuretic hormone (ADH). In infants, it often presents with:
Polyuria: Large volumes of dilute urine leading to severe dehydration.
Hypernatremia: High sodium levels due to dehydration.
Failure to thrive: Poor growth associated with diminished fluid intake.
Symptoms of dehydration: Vomiting, unexplained fever, irritability, and lethargy.
Management:
Hydration: Ensure adequate fluid intake to compensate for urinary losses.
Dietary modifications: A low-sodium, low-solute diet can help reduce urine output.
Medications: Hydrochlorothiazide and amiloride may be prescribed to enhance proximal tubular reabsorption of sodium and water, thereby reducing urine output. NSAIDs may also help in conjunction with thiazides.
Monitoring and support: Regular follow-up to ensure adequate hydration and manage long-term effects.
Hemolytic Uremic Syndrome (HUS)
Clinical Manifestations: HUS presents as a triad of:
Acute kidney injury (AKI)
Hemolytic anemia: Characterized by pallor and fatigue due to the destruction of red blood cells.
Thrombocytopenia: Low platelet count leading to bleeding tendencies such as bruising and purpura.
Patients often have a prodromal period of gastrointestinal illness (usually diarrhea) followed by rapid onset of the aforementioned symptoms. Severe cases may involve CNS symptoms, seizures, and renal failure.
Management:
Supportive care: Focus on fluid management, correction of electrolyte imbalances, and monitoring of vital signs.
Dialysis: Initiated for those who are anuric or severely oliguric with complications like hypertension or seizures.
Blood transfusions: For severe anemia, done cautiously to prevent fluid overload.
Nutritional support: Enteral or parenteral nutrition may be required if gastrointestinal symptoms are persistent.
Prognosis: With prompt treatment, survival rates are high, but some may experience long-term renal impairment.
Familial Nephritis (Alport Syndrome)
Clinical Manifestations: Alport syndrome is characterized by:
Hematuria: Presenting in infancy, often gross hematuria after respiratory infections.
Progressive renal failure: Typically develops in childhood with varying degrees of proteinuria.
Extrarenal manifestations: Sensorineural hearing loss and ocular abnormalities may also occur.
Management:
Supportive care: Management of blood pressure and proteinuria, often with ACE inhibitors.
Monitoring: Regular renal function tests to track progression.
End-stage renal disease (ESRD) management: Dialysis or kidney transplantation as ultimate interventions.
Genetic counseling: Important for affected families due to hereditary nature.
Unexplained Proteinuria
Clinical Manifestations: Children with unexplained proteinuria often appear healthy but may have:
Transient proteinuria: Commonly occurs during febrile illness, exercise, or dehydration.
Persistent proteinuria: May indicate underlying renal disease.
Orthostatic proteinuria: Common in adolescents, with protein levels higher in the standing position.
Management:
Diagnosis confirmation: Repeat urinalysis, orthostatic testing, and urine culture to exclude renal disease.
Further evaluation: If persistent, may require 24-hour protein excretion tests, renal ultrasound, or other imaging.
Monitoring: Regular follow-up to assess renal function and manage any identified renal pathology.
17. Discuss classifications of renal injury and therapeutic management of renal trauma in children.
18. Compare and contrast the pathophysiology, clinical manifestations, and nursing management of children with acute renal failure and with chronic renal failure (CRF).
Acute Kidney Injury (AKI) Overview
Definition: Acute Kidney Injury (AKI) occurs when the kidneys suddenly lose their ability to regulate urine volume and composition in response to food and fluid intake. Key features include oligoanuria (reduced urine output), azotemia (elevated nitrogenous waste in the blood), acidosis, and electrolyte imbalances. While not common in children, early recognition and treatment are crucial for favorable outcomes.
Etiology
AKI can arise from various clinical conditions, often classified into three categories:
Prerenal Causes:
Description: Related to reduced renal perfusion in a structurally normal kidney.
Common Causes:
Dehydration (from diarrhea or vomiting)
Surgical shock
Trauma (including burns)
Mechanism: Decreased renal perfusion leads to reduced glomerular filtration rate (GFR) and compensatory hormonal responses, potentially causing cortical or tubular necrosis if prolonged.
Intrinsic Renal Causes:
Description: Involves direct damage to the kidney tissues (glomeruli, tubules, or vasculature).
Common Causes:
Glomerular diseases (e.g., glomerulonephritis)
Tubular necrosis (ischemia or nephrotoxins)
Mechanism: Varies depending on the type and extent of damage, leading to varying degrees of renal dysfunction.
Postrenal Causes:
Description: Resulting from obstruction in the urinary tract.
Common Causes: Rare in children but may occur within the first year of life.
Mechanism: Relief of obstruction may restore function; duration of obstruction affects recovery.
Pathophysiology
Glomerular Filtration Rate (GFR): Severely reduced, leading to elevated Blood Urea Nitrogen (BUN) and altered sodium reabsorption.
Hormonal Responses: Increased renin secretion causes afferent arteriolar vasoconstriction, further reducing GFR.
Necrosis Types:
Cortical Necrosis: Severe ischemia or infections; most common in neonates.
Tubular Necrosis: Ischemia and nephrotoxic injuries, resulting in tubular epithelial cell death and possible incomplete recovery.
Clinical Course
Phases of AKI:
Oliguric Phase: Low urine output (<1 ml/kg/hr) with possible anuria.
Diuretic Phase: Abrupt increase in urine output, potentially leading to electrolyte imbalances.
Duration of the oliguric phase can vary significantly based on underlying causes.
Clinical Manifestations
Key Symptoms:
Oliguria or anuria
Edema
Drowsiness
Hypertension
Cardiac arrhythmias due to hyperkalemia
Neurologic symptoms (seizures, altered mental status)
Therapeutic Management
Prevention: Recognize at-risk situations and implement fluid therapy and nephrotoxin precautions.
Fluid and Electrolyte Management:
Restore volume for prerenal causes.
Monitor and restrict electrolytes based on levels and patient needs.
Management of Hyperkalemia:
Monitor serum potassium levels and ECG changes.
Temporary interventions: Calcium gluconate, sodium bicarbonate, insulin and glucose.
Definitive treatment: Sodium polystyrene sulfonate (Kayexalate) or dialysis.
Blood Pressure Control:
Monitor blood pressure every 4–6 hours.
Use antihypertensive medications (e.g., labetalol, sodium nitroprusside) as needed.
Nutritional Support:
Provide high-calorie, low-protein, low-potassium diets.
IV nutritional support if oral intake is insufficient.
Monitoring and Supportive Care:
Intensive monitoring of fluid balance, vital signs, and laboratory values.
Family support and education regarding the child's condition.
Prognosis
Outcomes: Dependent on the cause, severity, and timeliness of management. Mortality rates are generally less than 20%, with favorable outcomes expected in cases of dehydration or nephrotoxins, while HUS or rapidly progressive nephritis may lead to long-term complications.
Nursing Care Management
Focus on monitoring fluid and electrolyte balance, preventing complications, and providing family support.
Prepare for potential dialysis needs and ensure comprehensive care coordination in intensive settings.
Offer empathetic support to both child and family, addressing emotional and informational needs during treatment.
Chronic Kidney Disease (CKD) is a condition characterized by the progressive loss of kidney function over time, often leading to end-stage renal disease (ESRD), where renal replacement therapy, such as dialysis or transplantation, becomes necessary. The following details outline various aspects of CKD, including etiology, pathophysiology, clinical manifestations, diagnostic evaluation, and therapeutic management.
Etiology
The causes of CKD vary by age. In children under 5 years, the most common causes include:
Congenital renal and urinary tract malformations (e.g., renal hypoplasia, dysplasia, obstructive uropathy).
Vesicoureteral reflux (VUR).
For children aged 5 to 15 years, glomerular and hereditary renal diseases become more prevalent, including:
Chronic pyelonephritis.
Chronic glomerulonephritis (CGN).
Hereditary conditions such as Alport syndrome and congenital nephrotic syndrome.
Renal vascular disorders, although they are less common.
Pathophysiology
In the early stages of CKD, children often remain asymptomatic despite some biochemical changes. As nephron damage progresses:
The surviving nephrons undergo hypertrophy to maintain fluid and electrolyte balance.
As nephrons continue to be destroyed, uremic symptoms develop, marked by the retention of waste products, water, sodium, and imbalances in electrolytes and acids.
Key biochemical disturbances include:
Waste Product Retention: Elevated serum creatinine and BUN indicate declining kidney function.
Water and Sodium Retention: Resulting in edema and hypertension, especially in ESRD.
Hyperkalemia: Typically occurs in later stages due to the kidneys' inability to excrete potassium.
Metabolic Acidosis: A hallmark of CKD, due to impaired excretion of hydrogen ions and bicarbonate reabsorption.
Calcium and Phosphorus Disturbances: Can lead to renal osteodystrophy due to complex alterations in mineral metabolism.
Clinical Manifestations
Early signs of CKD may include:
Fatigue and decreased energy.
Pallor due to anemia.
Hypertension.
Growth impairment, observable on growth charts.
As the disease progresses, additional symptoms can manifest, including:
Decreased appetite and altered activity levels.
Urinary output changes (e.g., enuresis).
Symptoms of uremia, such as nausea, vomiting, and itching.
Neurologic symptoms like confusion or seizures may occur in advanced stages.
Diagnostic Evaluation
Diagnosis typically involves:
Clinical Assessment: Identification of symptoms and history of renal disease.
Laboratory Tests: Assessment of renal function through serum creatinine and BUN levels, urine analysis, and imaging studies as needed.
GFR Classification: Determining the stage of CKD based on the glomerular filtration rate (GFR).
Therapeutic Management
Management goals include maximizing renal function, maintaining fluid and electrolyte balance, treating systemic complications, and promoting normal development and quality of life. Key components include:
Activity
Encourage unrestricted activity tailored to the child's stamina.
Facilitate school attendance and home tutoring if necessary.
Diet
Tailored to provide adequate calories and protein while minimizing renal excretory demands.
May involve restrictions on protein, phosphorus, and sodium based on disease stage and symptoms.
Monitoring and supplementation of vitamins and minerals (excluding excess fat-soluble vitamins).
Osteodystrophy Management
Address calcium and phosphorus imbalances through diet, phosphorus-binding agents, and vitamin D supplementation.
Acidosis Treatment
Alkalizing agents like sodium bicarbonate to correct metabolic acidosis.
Anemia Management
Erythropoietin therapy to stimulate red blood cell production, coupled with iron supplementation.
Hypertension Management
Lifestyle modifications with dietary sodium restriction, fluid management, and pharmacological treatment as needed.
Growth and Development Support
Focus on nutrition, correction of imbalances, and potentially growth hormone therapy.
Monitoring for Complications
Regular assessments for intercurrent infections and other complications, adjusting medication dosages based on renal function.
Share
You said:
Discuss the clinical manifestations and management of the following renal diseases: nephrogenic diabetes insipidus, hemolytic uremic syndrome, familial nephritis (Alport syndrome), and unexplained proteinuria Renal Tubular Disorders Disorders of renal tubular function include a variety of conditions involving one or more abnormalities in specific mechanisms of tubular transport or reabsorption. Glomerular function is normal or mildly impaired. Eventually more widespread kidney destruction with renal failure may occur. In some cases the dysfunction has little, if any, effect on renal function. These disorders may be permanent or transient and may originate as primary defects or arise as a secondary effect of metabolic disease or exogenous toxins. Renal tubular disorders may be congenital (usually displaying characteristic patterns of genetic transmission), appear without evidence of hereditary transmission, or be acquired as a result of known or unknown causes. Unlike the classic manifestations of glomerular diseases, edema and hypertension are absent, and the BUN level and routine urinalysis are usually normal. Tubular proteinuria may be demonstrated. Manifestations of tubular disorders are primarily metabolic disturbances or deficiencies, such as failure to thrive, metabolic bone disease, or persistent acidosis. The variety of these disorders is extensive, and the incidence is rare. Tubular Function The function of the proximal tubules is the reabsorption of substances from the glomerular filtrate, including sodium, potassium, chloride, bicarbonate, glucose, phosphate, and amino acids. A number of disorders feature impairment of reabsorption of one or more filtrate constituents, and most involve defects in the transport mechanisms for these substances. Impaired tubular reabsorption of any specific substance causes that substance to appear in the urine, sometimes with reduced levels in the blood. Examples include bicarbonate and phosphate. The primary functions of the distal renal tubules are acidification of urine; potassium secretion; and selective and differential reabsorption of sodium, chloride, and water, which determines the final urinary concentration. Because the contribution of the distal tubule to urine composition depends in part on the volume and composition of the filtrate from the proximal tubule, the net contribution of the distal tubule is related to proximal tubular function and glomerular filtration. Renal Tubular Acidosis Renal tubular acidosis (RTA) is a syndrome of sustained metabolic acidosis in which there is impaired reabsorption of bicarbonate or excretion of net hydrogen ion but in which glomerular function is normal. On the basis of underlying pathophysiology, renal tubular acidosis is divided into proximal renal tubular acidosis and distal renal tubular acidosis. Proximal renal tubular acidosis results from a defect in bicarbonate reabsorption, whereas distal renal tubular acidosis results from an inability to establish an adequate gradient of pH between blood and tubular fluid. A number of genetic abnormalities have been identified for all types of primary RTA (Santos, Ordóñez, Claramunt-Taberner, et al., 2015). Proximal Tubular Acidosis (Type II) Impaired bicarbonate reabsorption in the proximal tubule causes proximal tubular acidosis. It may occur as an isolated defect (primary), but more often it appears in association with other proximal tubular disorders (secondary). As a result of a depressed renal threshold, bicarbonate reabsorption in the proximal tubule is incomplete, causing the plasma concentration of bicarbonate to stabilize at a lower level than normal. This results in a hyperchloremic metabolic acidosis. There is no impairment of distal tubular integrity or, in most cases, of the distal acidifying mechanism. A more complex abnormality in the proximal tubules is Fanconi syndrome, in which transport mechanisms are damaged by the accumulation of toxic metabolites or the tubular epithelium is damaged by heavy metals such as lead, cadmium, or platinum. Fanconi syndrome can be part of a number of hereditary diseases, be acquired, or be idiopathic (with a cause that is not identifiable). The major clinical manifestation and presenting symptom of Fanconi syndrome is growth failure. Tachypnea from hyperchloremic metabolic acidosis is also evident. Dehydration, vomiting, episodic fever, nephrolithiasis secondary to hypercalciuria, muscle weakness or paralysis as a result of hypokalemia, and episodes of severe life-threatening acidemia (sometimes triggered by a concurrent infection) may also be seen. The disorder may be transient or permanent. Distal Tubular Acidosis (Type I) Distal tubular acidosis is caused by the kidney's inability to establish a normal pH gradient between tubular cells and tubular contents. The most characteristic feature is the inability to produce a urinary pH below 6.0 despite the presence of severe metabolic acidosis. Distal renal tubular acidosis usually occurs as a primary, isolated defect but may also occur in association with other diseases or disorders (Gomez, Gil-Peña, & Santos, 2016). Most secondary causes are rare. The primary disorder is usually considered to be a hereditary defect with a variable degree of expression and a greater penetrance in females. After the age of 2 years, the child usually has growth failure, often with a history of vomiting, polyuria, dehydration, anorexia, and failure to thrive. Evidence of bone demineralization may be present, along with the occasional formation of renal calculi in older children. The inability to secrete hydrogen ions causes an accumulation of the ions in the body, which soon depletes the available hydrogen buffer and produces a sustained acidosis. Acidosis slows normal somatic growth, and demineralization of bone occurs as bone salts are mobilized to buffer the excessive hydrogen ions. Increased serum levels of both calcium and phosphorus contribute to the development of stones within the renal system. Both sodium and potassium are secreted in larger amounts. Serum potassium levels are depleted as the distal tubules excrete large amounts of potassium ions in an attempt to conserve sodium because hydrogen ions are unable to participate in the exchange. Hyponatremia stimulates increased aldosterone secretion, which further aggravates the hypokalemia. With the depletion of bicarbonate ions, more chloride is reabsorbed in the proximal tubule to create a hyperchloremia.
ChatGPT said:
ChatGPT
Let's break down the clinical manifestations and management of the renal diseases mentioned: nephrogenic diabetes insipidus, hemolytic uremic syndrome, familial nephritis (Alport syndrome), and unexplained proteinuria. Additionally, we'll touch on renal tubular disorders, particularly renal tubular acidosis (RTA), focusing on proximal and distal types.
1. Nephrogenic Diabetes Insipidus
Clinical Manifestations:
Polyuria: Excessive urination (3-20 liters per day) due to the kidney's inability to concentrate urine.
Polydipsia: Increased thirst, often leading to excessive water intake.
Dehydration: Symptoms include dry mouth, thirst, low blood pressure, and electrolyte imbalances.
Nocturia: Frequent urination at night.
Management:
Hydration: Ensuring adequate fluid intake to prevent dehydration.
Dietary changes: A low-protein, low-salt diet can help reduce urine output.
Medications:
Thiazide diuretics can paradoxically reduce urine output in nephrogenic DI.
Non-steroidal anti-inflammatory drugs (NSAIDs) may also reduce urine output.
Underlying cause treatment: Address any underlying conditions, such as chronic kidney disease or medications contributing to DI.
2. Hemolytic Uremic Syndrome (HUS)
Clinical Manifestations:
Triad of symptoms:
Hemolytic anemia: Characterized by fatigue, pallor, and jaundice.
Thrombocytopenia: Low platelet count leading to easy bruising and bleeding.
Acute renal failure: Symptoms may include decreased urine output, edema, and hypertension.
Gastrointestinal symptoms: Often associated with diarrhea (often bloody) due to Shiga toxin-producing E. coli.
Management:
Supportive care:
Fluid management and electrolyte balance are crucial.
Blood transfusions may be necessary for severe anemia.
Dialysis: May be required in cases of acute renal failure.
Avoidance of antibiotics and antidiarrheal medications: These can worsen the condition.
Monitoring: Close observation for complications, including hypertension and neurologic symptoms.
3. Familial Nephritis (Alport Syndrome)
Clinical Manifestations:
Hematuria: Blood in the urine, often an early sign.
Proteinuria: Presence of protein in urine, leading to nephrotic syndrome in advanced cases.
Progressive renal failure: Typically leads to end-stage renal disease by the second or third decade of life.
Ocular and auditory symptoms: Hearing loss and lens dislocation may occur due to mutations in collagen genes.
Management:
Blood pressure control: Use of ACE inhibitors can help slow progression of kidney disease.
Supportive care: Includes dietary modifications and management of symptoms.
Dialysis or transplant: May be required in cases of end-stage renal disease.
Genetic counseling: Important for affected families.
4. Unexplained Proteinuria
Clinical Manifestations:
Often asymptomatic but can lead to:
Edema
Hypertension
Fatigue or malaise if associated with nephrotic syndrome.
Management:
Assessment of underlying causes: Urinalysis, serum creatinine, and blood tests to rule out primary glomerular disease, infections, or systemic diseases (e.g., diabetes, lupus).
Monitor protein levels: Use urine protein-to-creatinine ratio for quantification.
Treat underlying conditions: Adjustments in management based on the identified cause (e.g., diabetes control, hypertension management).
Long-term follow-up: Regular monitoring of kidney function and proteinuria levels.
Renal Tubular Disorders
Renal Tubular Acidosis (RTA)
Proximal RTA (Type II)
Clinical Manifestations:
Metabolic acidosis with a normal anion gap.
Growth failure (especially in children), rickets, or osteomalacia.
Hypokalemia (low potassium).
Management:
Bicarbonate supplementation to correct acidosis.
Treatment of underlying causes, if secondary (e.g., medications or toxins).
Monitoring for bone health and addressing growth issues.
Distal RTA (Type I)
Clinical Manifestations:
Inability to acidify urine (urinary pH > 6.0).
Metabolic acidosis and growth retardation in children.
Risk of kidney stones and bone disease.
Management:
Bicarbonate supplementation to correct acidosis.
Potassium supplementation if hypokalemia occurs.
Monitoring of renal function and electrolytes.
Conclusion
Understanding the clinical manifestations and management strategies for these renal diseases is crucial in effectively diagnosing and treating patients. Regular monitoring and supportive care are vital to managing chronic conditions and preventing complications.
4o mini
You said:
Discuss the clinical manifestations and management of the following renal diseases: nephrogenic diabetes insipidus, hemolytic uremic syndrome, familial nephritis (Alport syndrome), and unexplained proteinuria Renal Tubular Disorders Disorders of renal tubular function include a variety of conditions involving one or more abnormalities in specific mechanisms of tubular transport or reabsorption. Glomerular function is normal or mildly impaired. Eventually more widespread kidney destruction with renal failure may occur. In some cases the dysfunction has little, if any, effect on renal function. These disorders may be permanent or transient and may originate as primary defects or arise as a secondary effect of metabolic disease or exogenous toxins. Renal tubular disorders may be congenital (usually displaying characteristic patterns of genetic transmission), appear without evidence of hereditary transmission, or be acquired as a result of known or unknown causes. Unlike the classic manifestations of glomerular diseases, edema and hypertension are absent, and the BUN level and routine urinalysis are usually normal. Tubular proteinuria may be demonstrated. Manifestations of tubular disorders are primarily metabolic disturbances or deficiencies, such as failure to thrive, metabolic bone disease, or persistent acidosis. The variety of these disorders is extensive, and the incidence is rare. Tubular Function The function of the proximal tubules is the reabsorption of substances from the glomerular filtrate, including sodium, potassium, chloride, bicarbonate, glucose, phosphate, and amino acids. A number of disorders feature impairment of reabsorption of one or more filtrate constituents, and most involve defects in the transport mechanisms for these substances. Impaired tubular reabsorption of any specific substance causes that substance to appear in the urine, sometimes with reduced levels in the blood. Examples include bicarbonate and phosphate. The primary functions of the distal renal tubules are acidification of urine; potassium secretion; and selective and differential reabsorption of sodium, chloride, and water, which determines the final urinary concentration. Because the contribution of the distal tubule to urine composition depends in part on the volume and composition of the filtrate from the proximal tubule, the net contribution of the distal tubule is related to proximal tubular function and glomerular filtration. Renal Tubular Acidosis Renal tubular acidosis (RTA) is a syndrome of sustained metabolic acidosis in which there is impaired reabsorption of bicarbonate or excretion of net hydrogen ion but in which glomerular function is normal. On the basis of underlying pathophysiology, renal tubular acidosis is divided into proximal renal tubular acidosis and distal renal tubular acidosis. Proximal renal tubular acidosis results from a defect in bicarbonate reabsorption, whereas distal renal tubular acidosis results from an inability to establish an adequate gradient of pH between blood and tubular fluid. A number of genetic abnormalities have been identified for all types of primary RTA (Santos, Ordóñez, Claramunt-Taberner, et al., 2015). Proximal Tubular Acidosis (Type II) Impaired bicarbonate reabsorption in the proximal tubule causes proximal tubular acidosis. It may occur as an isolated defect (primary), but more often it appears in association with other proximal tubular disorders (secondary). As a result of a depressed renal threshold, bicarbonate reabsorption in the proximal tubule is incomplete, causing the plasma concentration of bicarbonate to stabilize at a lower level than normal. This results in a hyperchloremic metabolic acidosis. There is no impairment of distal tubular integrity or, in most cases, of the distal acidifying mechanism. A more complex abnormality in the proximal tubules is Fanconi syndrome, in which transport mechanisms are damaged by the accumulation of toxic metabolites or the tubular epithelium is damaged by heavy metals such as lead, cadmium, or platinum. Fanconi syndrome can be part of a number of hereditary diseases, be acquired, or be idiopathic (with a cause that is not identifiable). The major clinical manifestation and presenting symptom of Fanconi syndrome is growth failure. Tachypnea from hyperchloremic metabolic acidosis is also evident. Dehydration, vomiting, episodic fever, nephrolithiasis secondary to hypercalciuria, muscle weakness or paralysis as a result of hypokalemia, and episodes of severe life-threatening acidemia (sometimes triggered by a concurrent infection) may also be seen. The disorder may be transient or permanent. Distal Tubular Acidosis (Type I) Distal tubular acidosis is caused by the kidney's inability to establish a normal pH gradient between tubular cells and tubular contents. The most characteristic feature is the inability to produce a urinary pH below 6.0 despite the presence of severe metabolic acidosis. Distal renal tubular acidosis usually occurs as a primary, isolated defect but may also occur in association with other diseases or disorders (Gomez, Gil-Peña, & Santos, 2016). Most secondary causes are rare. The primary disorder is usually considered to be a hereditary defect with a variable degree of expression and a greater penetrance in females. After the age of 2 years, the child usually has growth failure, often with a history of vomiting, polyuria, dehydration, anorexia, and failure to thrive. Evidence of bone demineralization may be present, along with the occasional formation of renal calculi in older children. The inability to secrete hydrogen ions causes an accumulation of the ions in the body, which soon depletes the available hydrogen buffer and produces a sustained acidosis. Acidosis slows normal somatic growth, and demineralization of bone occurs as bone salts are mobilized to buffer the excessive hydrogen ions. Increased serum levels of both calcium and phosphorus contribute to the development of stones within the renal system. Both sodium and potassium are secreted in larger amounts. Serum potassium levels are depleted as the distal tubules excrete large amounts of potassium ions in an attempt to conserve sodium because hydrogen ions are unable to participate in the exchange. Hyponatremia stimulates increased aldosterone secretion, which further aggravates the hypokalemia. With the depletion of bicarbonate ions, more chloride is reabsorbed in the proximal tubule to create a hyperchloremia. Prognosis. The primary disorder is usually permanent. However, secondary effects on growth and stone formation can be avoided with early diagnosis and therapy. When the disorder occurs as a secondary complication and renal damage is prevented, the prognosis is good (Gil-Peña, Mejía, & Santos, 2014). Therapeutic Management Treatment of both proximal and distal disorders consists of the administration of sufficient bicarbonate or citrate to balance metabolically produced hydrogen ions; to maintain the plasma bicarbonate level within normal range; and to correct associated electrolyte disorders, especially hypokalemia. Proximal disorders require large volumes of bicarbonate to compensate for urinary losses; in distal disorders the alkali required to maintain a normal plasma concentration is low. Most authorities favor a mixture of sodium and potassium bicarbonate (or citrate) to prevent deficiencies of either cation. The citrate solutions (Bicitra, Polycitra, or Shohl solution) are usually more easily tolerated than bicarbonate solutions. Nursing Care Management Nursing goals include recognizing the possibility of renal tubular acidosis in children who fail to thrive or who display other symptoms suggestive of the disorders and referring these children for medical evaluation. Helping parents understand the importance of adhering to the medication plan as a long-term goal is essential. (See Compliance and Administration of Medication, Chapter 22.) Children who must continue the medication indefinitely need to learn the importance of taking the medications as soon as they are old enough to assume responsibility for their own care. Nephrogenic Diabetes Insipidus Nephrogenic diabetes insipidus (NDI) is the major disorder associated with a defect in the ability to concentrate urine. In this disorder the distal tubules and collecting ducts are insensitive to the action of antidiuretic hormone or its exogenous counterpart, vasopressin. Although several inheritance patterns have been identified, more than 90% of patients have an X-linked defect of the vasopressin receptor (Bichet & Bockenhauer, 2016). The disease is more variable in female carriers of the defective gene, who may exhibit only a mild defect in urine-concentrating ability. The differential diagnosis for NDI should include chronic obstructive renal disorders, sickle cell disease, renal tuberculosis, and other renal disorders that may cause high urinary output with failure of the kidney to respond to vasopressin. Clinical Manifestations and Diagnostic Evaluation NDI is manifested in the newborn period by vomiting, unexplained fever, failure to thrive, and severe recurrent dehydration with hypernatremia. The passage of copious amounts of dilute urine, which produces severe dehydration and hypoelectrolytemia, is a serious threat to life during this period and may be responsible for the high incidence of cognitive impairment and motor retardation found in affected persons. Growth retardation is probably related to diminished food intake and poor general health because of uncontrolled polydipsia. Diagnosis is suspected on the basis of the patient and family history and confirmed by a urine osmolality value consistently below that of plasma. Lack of response to vasopressin administration rules out other causes. Therapeutic Management Therapy involves provision of adequate volumes of water to compensate for urinary losses and minimization of urine output through diet and medication. As a result of an insatiable thirst, most of the child's time is spent drinking and voiding, with decreased time for activity and stimulation. These children may go to great lengths to satisfy their thirst. A low-sodium, low-solute diet and the use of hydrochlorothiazide with or without amiloride to increase the reabsorption of sodium and water in the proximal tubule help reduce the amount of tubular fluid delivered to the distal tubules and to diminish the volume of water excreted (Dabrowski, Kadakia, & Zimmerman, 2016). Urinary output may be reduced when nonsteroidal antiinflammatory drugs (NSAIDs) are administered in conjunction with hydrochlorothiazide. Supplemental potassium may be required to prevent hypokalemia as a result of thiazide therapy. Normal growth and a normal life span are possible if the disease is recognized early and treatment is instituted and maintained. Nursing Care Management Nursing goals for children with NDI and their families are to recognize signs of the disorder early and assist them in coping with the long-term inconvenience of the continual thirst and elimination problems. Families need to learn to administer medications and help with diet planning for those on sodium restriction and needing supplemental potassium. The problem of ensuring adequate hydration is lifelong, and families need to adapt to away-from-home fluid needs and avoid activities that contribute to dehydration when fluids may not be available. Genetic counseling is recommended. Miscellaneous Renal Disorders Hemolytic Uremic Syndrome Hemolytic uremic syndrome (HUS) is an acute renal disease characterized by a triad of manifestations: AKI, hemolytic anemia, and thrombocytopenia. HUS occurs primarily in infants and small children between the ages of 6 months and 3 years. It has been recognized predominantly in Caucasians and, although it occurs worldwide, is more prevalent in South Africa, Argentina, and the west coasts of North and South America. HUS represents one of the main causes of AKI in early childhood. Etiology Diarrhea-positive (D+) HUS accounts for more than 90% of cases and is caused by ingestion of Shiga toxin producing Escherichia coli. E. coli O157:H7 is the most common pathogen, although other serotypes have also been reported (Karpman, Loos, Tati, et al., 2016). Occurrences tend to occur in scattered outbreaks and have been traced to undercooked meat, especially ground beef; unpasteurized apple juice; alfalfa sprouts; and public pools. Diarrhea negative (D−) or atypical HUS may be due to a number of causes, including nonenteric infections, disturbances in the complement system, malignancies, or genetic disorders (Loirat, Fakhouri, Ariceta, et al., 2016). Pathophysiology The primary site of injury appears to be the endothelial lining of the small glomerular arterioles, but other organs and tissues may be involved (e.g., the liver, brain, heart, pancreatic islet cells, and muscles). The endothelium becomes swollen and occluded with the deposition of platelets and fibrin clots (intravascular coagulation). Red blood cells are damaged as they move through the partially occluded blood vessels. The spleen removes these fragmented red blood cells, causing acute hemolytic anemia. Fibrinolytic action on the precipitated fibrin causes these fibrin-split products to appear in the serum and urine. The characteristic thrombocytopenia is produced by the platelet aggregation within damaged blood vessels or the damage and removal of platelets. Clinical Manifestations The disease occurs after a prodromal period, during which there is an episode of diarrhea and vomiting. Less often the preceding illness is an upper respiratory tract infection or, occasionally, varicella, measles, or a UTI. The hemolytic process persists for several days to 2 weeks. During this time the child is anorexic, irritable, and lethargic. There is marked and rapid onset of pallor accompanied by hemorrhagic manifestations such as bruising, purpura, or rectal bleeding. Severely affected patients are anuric and often hypertensive. Seizures and stupor suggest central nervous system involvement, and there may be signs of acute heart failure. Mild cases demonstrate anemia, thrombocytopenia, and azotemia; urinary output may be reduced or increased. Diagnostic Evaluation The triad of anemia, thrombocytopenia, and renal failure is sufficient for diagnosis. Proteinuria, hematuria, and urinary casts are evidence of renal involvement; BUN and serum creatinine levels are elevated. Low hemoglobin and hematocrit and a high reticulocyte count confirm the hemolytic nature of the anemia. Therapeutic Management Treatment is symptomatic and directed toward control of the complications and hematologic manifestations of renal failure. The initial supportive measures for most children are those used in managing renal failure: fluid replacement (calculated with great care), treatment of hypertension, and correction of acidosis and electrolyte disorders (Karpman, Loos, Tati, et al., 2016). The most consistently effective treatment is early hemodialysis, PD, or continuous hemofiltration, which is instituted in any child who has been anuric for 24 hours or who demonstrates oliguria with uremia or hypertension and seizures. Blood transfusions with fresh, washed packed cells are administered for severe anemia but are used with caution to prevent circulatory overload from added volume. Once vomiting and diarrhea have resolved, the child is restarted on enteral nutrition. Sometimes parenteral nutrition is required for children with severe, persistent colitis and for those in whom tissue catabolism is marked. There is no substantial evidence that heparin, corticosteroids, or fibrinolytic agents are beneficial, and in some instances they may aggravate the condition. Ecluzimab, a monoclonal antibody, has shown promise in treating and minimizing recurrences of D-HUS (Loirat, Fakhouri, Ariceta, et al., 2016). Prognosis. With prompt treatment the survival rate of D+ HUS is approximately 95% (Mody, Gu, Griffin, et al., 2015), but residual renal impairment ranges from 10% to 50%. Death is usually caused by residual renal impairment or central nervous system injury. D− HUS has a less favorable outcome, varying according to cause (Durkan, Kim, Craig, et al., 2016). Nursing Care Management Nursing care is the same as that provided in AKI and, for children with continued impairment, includes management of chronic disease. Because of the sudden and life-threatening nature of the disorder in a previously well child, parents are often ill prepared for the impact of hospitalization and treatment. Therefore support and understanding are especially important aspects of care. Nursing Alert To prevent infection from contaminated meat, the internal temperature of the food, such as hamburger, should be at least 74°C (165°F). Cooking the ground beef until no pink color is seen may not be sufficient to kill the bacteria. Therefore a meat thermometer is needed to ensure a safe product. Discourage parents from giving children unpasteurized apple juice and unwashed raw vegetables. Also discourage the use of antimotility drugs for diarrhea. Familial Nephritis (Alport Syndrome) Alport syndrome (AS) is a hereditary disease characterized by high-tone sensorineural deafness, ocular disorders, and chronic kidney disease caused by mutations in type IV collagen. Most people with AS have the X-linked form of the condition. Less common are autosomal recessive and dominant forms. Hematuria presents during infancy in affected boys. Gross hematuria may be associated with acute respiratory tract infections. Proteinuria and progressive renal failure begin in childhood. The progression rate to end-stage kidney disease depends on the form of AS. Females may have only microhematuria or progress to ESRD, again depending on the form of the condition they have (Savige, Colville, Rheault, et al., 2016). Treatment is symptomatic and supportive. Dialysis and kidney transplantation are ultimate therapeutic measures for ESRD. Hearing loss and ocular disorders should receive appropriate attention, and families should be counseled regarding the genetic implications of the disease. Unexplained Proteinuria Often apparently healthy children with no suggestion of renal disease demonstrate proteinuria on routine urinalysis. The percentage of children with unexplained proteinuria ranges from 1% at 6 years of age to 11% at puberty, reaching a maximum prevalence at age 13 in girls and age 16 in boys. Unexplained proteinuria can be categorized as transient (inconstant), persistent, or orthostatic or postural. Transient proteinuria is a common finding with no known cause but sometimes increases with febrile illness, exercise, or dehydration. Persistent proteinuria usually signifies renal disease. Orthostatic proteinuria is seen in 3% to 5% of adolescents and young adults; although proteinuria is evident in both the recumbent and the erect position, it is quantitatively greater in the erect position. The cause is unknown, but minor glomerular changes occur in many instances. The condition is benign and generally resolves over time. In cases of unexplained proteinuria, it is important to confirm or exclude renal disease with appropriate diagnostic tests. Repeated examination for proteinuria, an orthostatic test, urine culture, and (if proteinuria is persistent) more definitive tests—including 24-hour protein excretion, renal ultrasound, and renal scan—are indicated.
ChatGPT said:
ChatGPT
Here's a discussion on the clinical manifestations and management of nephrogenic diabetes insipidus (NDI), hemolytic uremic syndrome (HUS), familial nephritis (Alport syndrome), and unexplained proteinuria, focusing on their renal implications:
Nephrogenic Diabetes Insipidus (NDI)
Clinical Manifestations: NDI is characterized by the kidneys' inability to concentrate urine due to insensitivity to antidiuretic hormone (ADH). In infants, it often presents with:
Polyuria: Large volumes of dilute urine leading to severe dehydration.
Hypernatremia: High sodium levels due to dehydration.
Failure to thrive: Poor growth associated with diminished fluid intake.
Symptoms of dehydration: Vomiting, unexplained fever, irritability, and lethargy.
Management:
Hydration: Ensure adequate fluid intake to compensate for urinary losses.
Dietary modifications: A low-sodium, low-solute diet can help reduce urine output.
Medications: Hydrochlorothiazide and amiloride may be prescribed to enhance proximal tubular reabsorption of sodium and water, thereby reducing urine output. NSAIDs may also help in conjunction with thiazides.
Monitoring and support: Regular follow-up to ensure adequate hydration and manage long-term effects.
Hemolytic Uremic Syndrome (HUS)
Clinical Manifestations: HUS presents as a triad of:
Acute kidney injury (AKI)
Hemolytic anemia: Characterized by pallor and fatigue due to the destruction of red blood cells.
Thrombocytopenia: Low platelet count leading to bleeding tendencies such as bruising and purpura.
Patients often have a prodromal period of gastrointestinal illness (usually diarrhea) followed by rapid onset of the aforementioned symptoms. Severe cases may involve CNS symptoms, seizures, and renal failure.
Management:
Supportive care: Focus on fluid management, correction of electrolyte imbalances, and monitoring of vital signs.
Dialysis: Initiated for those who are anuric or severely oliguric with complications like hypertension or seizures.
Blood transfusions: For severe anemia, done cautiously to prevent fluid overload.
Nutritional support: Enteral or parenteral nutrition may be required if gastrointestinal symptoms are persistent.
Prognosis: With prompt treatment, survival rates are high, but some may experience long-term renal impairment.
Familial Nephritis (Alport Syndrome)
Clinical Manifestations: Alport syndrome is characterized by:
Hematuria: Presenting in infancy, often gross hematuria after respiratory infections.
Progressive renal failure: Typically develops in childhood with varying degrees of proteinuria.
Extrarenal manifestations: Sensorineural hearing loss and ocular abnormalities may also occur.
Management:
Supportive care: Management of blood pressure and proteinuria, often with ACE inhibitors.
Monitoring: Regular renal function tests to track progression.
End-stage renal disease (ESRD) management: Dialysis or kidney transplantation as ultimate interventions.
Genetic counseling: Important for affected families due to hereditary nature.
Unexplained Proteinuria
Clinical Manifestations: Children with unexplained proteinuria often appear healthy but may have:
Transient proteinuria: Commonly occurs during febrile illness, exercise, or dehydration.
Persistent proteinuria: May indicate underlying renal disease.
Orthostatic proteinuria: Common in adolescents, with protein levels higher in the standing position.
Management:
Diagnosis confirmation: Repeat urinalysis, orthostatic testing, and urine culture to exclude renal disease.
Further evaluation: If persistent, may require 24-hour protein excretion tests, renal ultrasound, or other imaging.
Monitoring: Regular follow-up to assess renal function and manage any identified renal pathology.
Conclusion
These renal disorders highlight the diverse manifestations and management strategies required for effective patient care. Early recognition and intervention are crucial to prevent complications and improve long-term outcomes in affected individuals.
4o mini
You said:
Discuss classifications of renal injury and therapeutic management of renal trauma in children. Acute Kidney Injury AKI is said to exist when the kidneys suddenly are unable to appropriately regulate the volume and composition of urine in response to food and fluid intake and the needs of the organism. The principal feature is oligoanuria* associated with azotemia, acidosis, and diverse electrolyte disturbances. AKI is not common in childhood. The outcome depends on the cause, associated findings, and prompt recognition and treatment. Etiology AKI can develop as a result of a large number of related or unrelated clinical conditions: poor renal perfusion, acute renal injury, or the final expression of chronic renal disease. The most common cause in children is transient renal failure resulting from dehydration or other causes of poor perfusion that respond to restoration of fluid volume. Causes of AKI are usually classified as prerenal, intrinsic renal, and postrenal. Severe or long-standing prerenal or postrenal causes can produce severe secondary renal damage. Prerenal Causes. Prerenal causes of AKI are most common in children and are related to the reduction of renal perfusion in an anatomically and physiologically normal kidney and collecting system. Dehydration secondary to diarrheal disease or persistent vomiting is the most common cause of prerenal failure in infants and children. Surgical shock and trauma (including burns) are also common causes. Hypovolemia and decreased renal perfusion cause a decreased glomerular filtration rate and stimulate the secretion of renin, aldosterone, and antidiuretic hormone, which further diminish urine flow. Extended and severe hypoperfusion (secondary to procedures such as cardiac surgery) can produce cortical or tubular necrosis. Increasing awareness of the potential for the development of AKI allows for earlier detection and treatment should it occur (Jefferies & Devarajan, 2016). In general, the azotemia that accompanies this type of renal failure is rapidly reversible with prompt attention to expansion of the extracellular fluid volume. Prerenal failure is often difficult to distinguish from tubular or cortical necrosis. Renal artery stenosis, altered peripheral vascular resistance related to sepsis, and hepatorenal syndrome are less common causes. Intrinsic Renal Causes. Intrinsic renal causes of AKI constitute the largest group that requires extended management. These include diseases and nephrotoxic agents that damage the glomeruli, tubules, or renal vasculature. Glomerular disease is the most common cause of glomerular damage, whereas tubular destruction is more often caused by ischemia or nephrotoxins. Vascular damage is an uncommon cause of renal failure in childhood. The type and extent of damage determine the degree and duration of renal insufficiency, and it is difficult to predict in any given case whether acute necrosis will develop. Postrenal Causes. AKI resulting from obstructive uropathy is uncommon in children except during the first year of life. Relief of the obstruction can restore renal function. The degree of recovery depends on the duration of the renal failure. Pathophysiology AKI is usually reversible, but the deviations of physiologic function can be extreme, and mortality in the pediatric age-group is still high. There is severe reduction in the glomerular filtration rate, an elevated BUN level, and decreased tubular reabsorption of sodium from the proximal tubule. Consequently, there is increased concentration of sodium in the distal tubule, which causes stimulation of the renin mechanism. The local action of angiotensin causes vasoconstriction of the afferent arteriole, which further reduces glomerular filtration and prevents urinary losses of sodium. There is a significant reduction in renal blood flow. The pathologic conditions that produce AKI caused by glomerulonephritis, HUS, and other renal disorders are discussed in relation to those disease processes. The necrotic processes within the nephron can be cortical, tubular, or both. Cortical Necrosis. Complete cortical necrosis usually results from severe ischemia, infection, or intravascular coagulation and represents a severe cause of AKI. In the pediatric age-group this occurs most commonly during the neonatal period as a result of hypoxia and shock. When cortical destruction is incomplete, some recovery of renal function may occur. Tubular Necrosis. Damage to the renal tubules can be broadly classified as secondary to renal ischemia and associated with the ingestion or inhalation of substances toxic to the kidneys. Renal tubules are particularly vulnerable to a wide variety of toxic agents that produce vasoconstriction and to focal patches of ischemia that cause a necrosis of the tubular epithelium down to, but not including, the basement membrane. A lesion produced by sustained reduction in renal blood flow also involves the basement membrane, which may become fragmented and ruptured to the extent that the continuity of tubular structure is disrupted. The lesions may affect any segment of the tubules, appearing at irregular intervals along with normal segments throughout the kidney. Reepithelialization in the areas with intact basement membrane heals tubular lesions. Such healing is unable to take place in areas in which the basement membrane has been disrupted; connective tissue grows through the ruptured membrane, thus preventing reestablishment of tubular integrity. Individual cells within the nephron, but not the entire nephron, are capable of regeneration. Clinical Course. The clinical course of the child with AKI is variable and depends on the cause. In reversible AKI there is a period of severe oliguria, or a low-output phase, followed by an abrupt onset of diuresis, or a high-output phase; this phase is followed by a gradual return to, or toward, normal urine volumes. The length of the oliguric phase in older children and adolescents is 10 to 14 days but is highly variable at all ages depending on the cause of the AKI. The onset of the diuretic phase appears unexpectedly, and over several days it proceeds in stepwise fashion from very low to above-normal urine volumes. During the oliguric phase, manifestations of uremia are present but may also be accompanied by other clinical disorders that make assessment difficult, such as infection, anoxia, and shock. Clinical Manifestations In many instances of AKI the infant or child is already critically ill with the precipitating disorder, and the explanation for development of oliguria may or may not be readily apparent. The underlying illness often overshadows the renal failure and often assumes the priority of care (e.g., the patient who is in shock from endotoxemia, the infant who is severely dehydrated from gastroenteritis, or a child who is subject to seizures as a result of hypertensive encephalopathy associated with AGN). The prime manifestation of AKI is oliguria, generally a urinary output of less than 1 ml/kg/hr. Anuria (no urinary output in 24 hours) is uncommon, except in obstructive disorders. Other symptoms related to AKI include edema, drowsiness, circulatory congestion, and cardiac arrhythmia from hyperkalemia. Seizures may be caused by hyponatremia or hypocalcemia and tachypnea from metabolic acidosis. With continued oliguria, biochemical abnormalities can develop rapidly, and circulatory and central nervous system manifestations appear. Diagnostic Evaluation When a previously well child develops AKI without obvious cause, a careful history is obtained to reveal symptoms that may be related to glomerulonephritis; obstructive uropathy; or exposure to nephrotoxic chemicals, such as ingestion of heavy metals or inhalation of carbon tetrachloride or other organic solvents or drugs (e.g., methicillin, sulfonamides, NSAIDs, neomycin, polymyxin, and kanamycin). Laboratory data reflect the kidney dysfunction: hyperkalemia, hyponatremia, metabolic acidosis, hypocalcemia, anemia, or azotemia (Table 24.7). TABLE 24.7 Laboratory Findings Associated With Acute Renal Failure Clinical Problem Mechanism Clinical Considerations Azotemia Ongoing protein catabolism Lower rate of production in neonates and persons with depleted protein stores Elevated blood urea nitrogen levels Significantly decreased excretion Increased in situations involving large amounts of necrotic tissue or extravasated blood Elevated plasma creatinine levels Continued production Significantly decreased excretion Production less affected by other factors More sensitive measure of intensity of azotemia Low in neonate because of small muscle mass relative to size Metabolic acidosis Continued endogenous acid production Significantly decreased excretion Depletion of extracellular and intracellular fluid buffers Compensatory hyperventilation Opisthonos Major threat to life Hyponatremia Dilution of extracellular fluid Decreased excretion of water May develop cerebral signs Hyperkalemia Ongoing protein catabolism Decreased excretion compounded by metabolic acidosis Most important electrolyte to be considered in acute renal failure May contribute to cardiac arrhythmia With electrocardiogram changes, major threat to life Loss may be from gastrointestinal tract Hypocalcemia Associated with metabolic acidosis and hyperphosphatemia During alkali therapy, may cause tetany Therapeutic Management The most effective management of AKI is prevention. The development of AKI is a known risk in certain situations. This should be anticipated and recognized, and adequate therapy should be implemented (e.g., fluid therapy for children with hypovolemia in conditions such as dehydration, burns, and hemorrhage). Nephrotoxic drugs should be used with caution or avoided in children with renal disease, and all personnel should be knowledgeable about precautions related to their administration. For example, a generous fluid intake is needed for children receiving antimetabolite drugs and after radiotherapy. The treatment of AKI is directed toward treatment of the underlying cause, management of the complications of renal failure, and provision of supportive therapy within the constraints imposed by the renal failure. Treatment of poor perfusion resulting from dehydration consists of volume restoration as described in the treatment of dehydration. (See Chapter 23.) If oliguria persists after restoration of fluid volume or if the renal failure is caused by intrinsic renal damage, the physiologic and biochemical abnormalities that have resulted from kidney dysfunction must be corrected or controlled. Central venous pressure monitoring is usually implemented. Initially a catheter is inserted to rule out urine retention, to collect available urine for electrolytes and analysis, and to monitor the results of diuretic administration. The catheter may or may not be removed. Some clinicians believe that it serves little purpose during the oliguric phase and predisposes the patient to bladder infections. Others maintain a catheter for hourly urine measurements. The use of mannitol, loop diuretics such as furosemide and other medications in the prevention and treatment of AKI have been studied. The 2012 KDIGO Guidelines on Acute Kidney Injury state that the use of mannitol in the prevention of AKI is not scientifically justified due to inadequate studies. They do not recommend the use of diuretics to prevent or treat AKI except as an aid in the management of volume overload. Fluid and Calories. The amount of exogenous water provided should not exceed the amount needed to maintain zero water balance. It is calculated on the basis of estimated endogenous water formation and losses from sensible (primarily gastrointestinal) and insensible sources. No allotment is calculated for urine as long as oliguria persists. The child with AKI has a tendency to develop water intoxication and hyponatremia, both of which make it difficult to provide calories in sufficient amounts to meet the child's needs and reduce tissue catabolism, metabolic acidosis, hyperkalemia, and uremia. If the child is able to tolerate oral foods, concentrated food sources that are high in carbohydrates and fat but low in protein, potassium, and sodium may be provided. However, many children have functional disturbances of the gastrointestinal tract, such as nausea and vomiting. Therefore the IV route is generally preferred, and nourishment usually consists of essential amino acids or a combination of essential and nonessential amino acids administered by the central venous route. Control of water balance in these patients requires careful monitoring of feedback information, such as accurate intake and output, body weight, and electrolyte measurements. In general, during the oliguric phase, no sodium, chloride, or potassium is given unless there are other large, ongoing losses. Regular measurement of plasma electrolytes, pH, BUN, and creatinine levels is required to assess the adequacy of fluid therapy and to anticipate complications that require specific treatment. Hyperkalemia. An elevated serum potassium level is the most immediate threat to the life of the child with AKI. Potassium ions are not being excreted, while at the same time the release of potassium from cells is accelerated by acidosis, stress, and tissue breakdown in cases associated with internal bleeding or trauma. Because cardiac arrhythmia and cardiac arrest may result, electrocardiograms (ECGs) and serum potassium ion levels are monitored regularly. Hyperkalemia can be minimized and sometimes avoided by eliminating potassium from all food and fluids, by reducing tissue catabolism, and by correcting acidosis. Nursing Alert Any of the following signs of hyperkalemia constitute an emergency and should be reported immediately: • Serum potassium concentrations in excess of 7 mEq/L • Presence of ECG abnormalities, such as loss of P wave, prolonged RS complex, depressed ST segment, tall and tented T waves, bradycardia, or heart block Several measures are available to reduce the serum potassium concentration, and the priority of implementation is usually based on the rapidity with which the measures are effective. Temporary measures that produce a rapid but transient effect are as follows: • Calcium gluconate administered intravenously over 2 to 4 minutes with continuous ECG monitoring, exerts a protective effect on cardiac conduction. • Sodium bicarbonate administered intravenously over 30 to 60 minutes, elevates the serum pH to cause a transient shift of extracellular fluid potassium into the intracellular fluid. However, there is a risk of hypocalcemia, tetany, and fluid overload. • Glucose and insulin administered intravenously, accelerate glycogen synthesis, causing glucose and potassium to move into the cells. Insulin facilitates the entry of glucose into cells. These effects produce only transient protection by redistributing existing potassium stores; they do not remove potassium from the body. However, they provide relief while more definitive but slower-acting measures are being implemented. Potassium can be removed by either of two methods: 1. Administration of a cation exchange resin such as sodium polystyrene sulfonate (Kayexalate), 1 g/kg, administered orally or rectally, to bind potassium and remove it from the body. This requires time to be effective, and a sodium ion is exchanged for each potassium ion. This increased sodium concentration adds to the body fluids, which may contribute to fluid overload, hypertension, and cardiac failure. 2. Dialysis or continuous hemofiltration (see p. 810). Hemodialysis is efficient but requires specialized facilities. PD is simpler and can be carried out in almost any hospital setting. Indications for dialysis in AKI are continued oliguria associated with any of the following: • Severe, persistent acidosis • Inability to reduce serum potassium levels to a safe range with other methods • Clinical uremic syndrome consisting of nausea and vomiting, drowsiness, and progression to coma • Circulatory overload, hypertension, and evidence of cardiac failure The optimal timing for initiation of renal replacement therapy is controversial. One strategy is to institute renal replacement therapy within hours of the diagnosis of severe AKI being made, regardless of other symptoms. Another strategy is to delay renal replacement therapy until any of the previously listed symptoms presents. Differences in mortality have not been demonstrated between the two approaches (Gaudry, Hajage, Schortgen, et al., 2016). Hypertension. Hypertension is a common and serious complication of AKI, and blood pressure determinations are taken at least every 4 to 6 hours to detect it early. The most common cause of hypertension in AKI is overexpansion of the extracellular fluid and plasma volume, together with activation of the renin-angiotensin system. The goal of therapy is to prevent hypertensive encephalopathy and avoid overtaxing the cardiovascular system. When there is a threat of encephalopathy, labetalol (a beta and alpha blocker) may be administered intravenously as bolus infusions or a continuous drip. Sodium nitroprusside may be given but requires close monitoring. For less urgent situations, hydralazine, clonidine, or verapamil may be given intravenously. Oral drugs used for acute hypertension include nifedipine, captopril, minoxidil, hydralazine, propranolol, or furosemide. Other Complications. Other complications that may occur with AKI are anemia, seizures and coma, cardiac failure, and pulmonary edema. Anemia is commonly associated with AKI, but transfusion is not recommended unless the hemoglobin level drops below 6 g/dl. Transfusions consist of fresh, packed red blood cells given slowly to reduce the likelihood of increasing blood volume, hypertension, and hyperkalemia. Seizures occur often when renal failure progresses to uremia and are also related to hypertension, hyponatremia, and hypocalcemia. Treatment is directed toward the specific cause when known. More obscure causes are managed with antiepileptic drugs. Cardiac failure with pulmonary edema is almost always associated with hypervolemia. Treatment is directed toward reduction of fluid volume, with water and sodium restriction and administration of diuretics. Digitalis is ineffective and can be hazardous. Diuretic, or High-Output, Phase. When the output begins to increase, either spontaneously or in response to diuretic therapy, the nurse should monitor the intake of fluid, potassium, and sodium, and provide adequate replacement to prevent depletion and its consequences. In some cases the high-output phase is mild and lasts only a few days; in others enormous amounts of electrolyte-rich urine are passed. Prognosis. The prognosis of AKI depends largely on the nature and severity of the causative factor or precipitating event and the promptness and competence of management. The mortality rate is less than 20%. The outcome is least favorable in children with rapidly progressive nephritis and cortical necrosis. Children in whom AKI is a result of HUS or AGN may recover completely, but residual renal impairment or hypertension is more often the rule. Complete recovery is usually expected in children whose renal failure is a result of dehydration, nephrotoxins, or ischemia. AKI after cardiac surgery has a less favorable prognosis. It is often impossible to assess the extent of recovery for several months. (See Quality Patient Outcomes box.) Quality Patient Outcomes Acute Kidney Injury • Underlying cause of acute kidney injury identified and treated • Water balance maintained • Hypertension controlled • Electrolyte balance maintained • Diet maintains calories while minimizing tissue catabolism, metabolic acidosis, hyperkalemia, and uremia Nursing Care Management Nursing care of the infant or child with AKI involves addressing the underlying cause plus carefully observing and managing the renal status. The major goal is reestablishment of renal function (with emphasis on providing an adequate caloric intake to minimize reduction of protein stores); prevention of complications; and monitoring of fluid balance, laboratory data, and physical manifestations. The probability of dialysis or continuous hemofiltration is high, and the nurse must anticipate the availability of the necessary equipment. Because the child requires intensive observation and often specialized equipment, the usual disposition is admission to an intensive care unit where equipment and trained personnel are available. The major nursing tasks in the care of the infant or child with AKI are monitoring and assessing fluid and electrolyte balance. Limiting fluid intake requires ingenuity on the part of caregivers to cope with the child who is thirsty. One strategy involves rationing the daily intake with small amounts of fluid served in containers that give the impression of larger volumes. Older children who understand the rationale of fluid limits can help determine how their daily ration should be distributed. Meeting nutritional needs is sometimes a problem because the child may be nauseated and because getting the child to eat concentrated foods without fluids may be difficult. When nourishment is provided by the IV route, careful monitoring is essential to prevent fluid overload. This can become a major challenge in the face of nutritional requirements and administration of IV medications. The IV drugs being used may be nephrotoxic, which can require a specified volume of solution for delivery. In some instances blood products must also be delivered. Preventing fluid overload while delivering medications and calories requires concerted collaboration. In addition, nursing measures such as maintaining an optimum thermal environment, reducing any elevation of body temperature, and reducing restlessness and anxiety are used to decrease the rate of tissue catabolism. The nurse must be continually alert for behavior changes that indicate the onset of complications. Infection from reduced resistance, anemia, and general morbidity is a constant threat. Fluid overload and electrolyte disturbances can precipitate cardiovascular complications such as hypertension and cardiac failure. Fluid and electrolyte imbalances, acidosis, and accumulation of nitrogenous waste products can produce neurologic involvement manifested by coma, seizures, or alterations in sensorium. Although children with AKI are usually quite ill and voluntarily diminish their activity, infants may become restless and irritable, and children are often anxious and frightened. Frequent, painful, and stress-producing treatments and tests must be performed. A supportive, empathetic nurse can provide comfort and stability in a threatening and unnatural environment. Family Support. Providing support and reassurance to parents is among the major nursing responsibilities. The seriousness and emergency nature of AKI are stressful to parents, and most feel some degree of guilt regarding the child's condition, especially when the illness is the result of ingestion of a toxic substance, dehydration, or a genetic disease. They need reassurance and an empathetic listener. They also need to be kept informed of the child's progress and provided explanations regarding the therapeutic regimen. The equipment and the child's behavior are sometimes frightening and anxiety provoking. Nurses can do much to help parents comprehend and deal with the stresses of the situation.
ChatGPT said:
ChatGPT
Classifications of Renal Injury in Children
Renal injuries in children can be classified based on the mechanisms that cause the injury and their clinical presentation. The primary classifications include:
Acute Kidney Injury (AKI):
Prerenal AKI: This type occurs due to decreased renal perfusion and is the most common cause in children. Causes include dehydration from diarrhea, vomiting, or surgical shock.
Intrinsic Renal AKI: This occurs from direct damage to the kidney tissues, which can be due to glomerular diseases, acute tubular necrosis from ischemia or nephrotoxins, or vascular issues.
Postrenal AKI: This type is caused by obstructive uropathy, which is less common in children but can occur in the neonatal period.
Chronic Kidney Disease (CKD): While not an acute event, chronic kidney conditions can lead to long-term renal injury, affecting a child’s growth and development.
Traumatic Kidney Injury: Renal injuries due to trauma can range from minor contusions to lacerations and vascular injuries. Common causes in children include falls, sports injuries, or blunt trauma.
Therapeutic Management of Renal Trauma in Children
Management of renal trauma requires a systematic approach, often involving both conservative and surgical interventions. Here are key aspects:
1. Initial Assessment and Stabilization
Evaluate Hemodynamics: Monitor blood pressure, heart rate, and perfusion status.
History and Physical Examination: Obtain details regarding the injury mechanism and assess for signs of renal injury, such as hematuria or abdominal pain.
Imaging Studies: Use ultrasound or CT scans to determine the extent of kidney damage and assess for urinary tract injuries.
2. Conservative Management
Observation: Many renal injuries, particularly minor ones, can be managed conservatively. This involves close monitoring of renal function and vital signs.
Fluid Resuscitation: Address hypovolemia with IV fluids as needed. This is critical if there are signs of shock.
Pain Management: Provide adequate analgesia to manage discomfort associated with the injury.
Avoid Nephrotoxic Agents: Reduce the use of medications that can worsen renal function.
3. Surgical Management
Indications for Surgery: Surgical intervention may be required for significant renal lacerations, vascular injuries, or renal pedicle injuries. Other indications include persistent bleeding or failure to respond to conservative management.
Surgical Techniques: Depending on the injury's severity, procedures may range from nephrectomy (removal of the kidney) to renal repair or arterial embolization to control bleeding.
4. Post-Management Monitoring
Renal Function Monitoring: Regularly check serum creatinine and electrolyte levels to evaluate renal function recovery.
Complication Management: Monitor for complications such as urinary obstruction or hypertension, which may require further intervention.
Family Support and Education: Provide emotional support to the family and educate them about the child's condition, treatment options, and recovery process.
Conclusion
Renal injury in children, particularly AKI, requires prompt recognition and appropriate management to minimize long-term complications. Both conservative and surgical approaches may be utilized depending on the severity and nature of the injury. Close monitoring and supportive care are essential components of managing these pediatric renal injuries effectively.
4o mini
You said:
Acute Kidney Injury AKI is said to exist when the kidneys suddenly are unable to appropriately regulate the volume and composition of urine in response to food and fluid intake and the needs of the organism. The principal feature is oligoanuria* associated with azotemia, acidosis, and diverse electrolyte disturbances. AKI is not common in childhood. The outcome depends on the cause, associated findings, and prompt recognition and treatment. Etiology AKI can develop as a result of a large number of related or unrelated clinical conditions: poor renal perfusion, acute renal injury, or the final expression of chronic renal disease. The most common cause in children is transient renal failure resulting from dehydration or other causes of poor perfusion that respond to restoration of fluid volume. Causes of AKI are usually classified as prerenal, intrinsic renal, and postrenal. Severe or long-standing prerenal or postrenal causes can produce severe secondary renal damage. Prerenal Causes. Prerenal causes of AKI are most common in children and are related to the reduction of renal perfusion in an anatomically and physiologically normal kidney and collecting system. Dehydration secondary to diarrheal disease or persistent vomiting is the most common cause of prerenal failure in infants and children. Surgical shock and trauma (including burns) are also common causes. Hypovolemia and decreased renal perfusion cause a decreased glomerular filtration rate and stimulate the secretion of renin, aldosterone, and antidiuretic hormone, which further diminish urine flow. Extended and severe hypoperfusion (secondary to procedures such as cardiac surgery) can produce cortical or tubular necrosis. Increasing awareness of the potential for the development of AKI allows for earlier detection and treatment should it occur (Jefferies & Devarajan, 2016). In general, the azotemia that accompanies this type of renal failure is rapidly reversible with prompt attention to expansion of the extracellular fluid volume. Prerenal failure is often difficult to distinguish from tubular or cortical necrosis. Renal artery stenosis, altered peripheral vascular resistance related to sepsis, and hepatorenal syndrome are less common causes. Intrinsic Renal Causes. Intrinsic renal causes of AKI constitute the largest group that requires extended management. These include diseases and nephrotoxic agents that damage the glomeruli, tubules, or renal vasculature. Glomerular disease is the most common cause of glomerular damage, whereas tubular destruction is more often caused by ischemia or nephrotoxins. Vascular damage is an uncommon cause of renal failure in childhood. The type and extent of damage determine the degree and duration of renal insufficiency, and it is difficult to predict in any given case whether acute necrosis will develop. Postrenal Causes. AKI resulting from obstructive uropathy is uncommon in children except during the first year of life. Relief of the obstruction can restore renal function. The degree of recovery depends on the duration of the renal failure. Pathophysiology AKI is usually reversible, but the deviations of physiologic function can be extreme, and mortality in the pediatric age-group is still high. There is severe reduction in the glomerular filtration rate, an elevated BUN level, and decreased tubular reabsorption of sodium from the proximal tubule. Consequently, there is increased concentration of sodium in the distal tubule, which causes stimulation of the renin mechanism. The local action of angiotensin causes vasoconstriction of the afferent arteriole, which further reduces glomerular filtration and prevents urinary losses of sodium. There is a significant reduction in renal blood flow. The pathologic conditions that produce AKI caused by glomerulonephritis, HUS, and other renal disorders are discussed in relation to those disease processes. The necrotic processes within the nephron can be cortical, tubular, or both. Cortical Necrosis. Complete cortical necrosis usually results from severe ischemia, infection, or intravascular coagulation and represents a severe cause of AKI. In the pediatric age-group this occurs most commonly during the neonatal period as a result of hypoxia and shock. When cortical destruction is incomplete, some recovery of renal function may occur. Tubular Necrosis. Damage to the renal tubules can be broadly classified as secondary to renal ischemia and associated with the ingestion or inhalation of substances toxic to the kidneys. Renal tubules are particularly vulnerable to a wide variety of toxic agents that produce vasoconstriction and to focal patches of ischemia that cause a necrosis of the tubular epithelium down to, but not including, the basement membrane. A lesion produced by sustained reduction in renal blood flow also involves the basement membrane, which may become fragmented and ruptured to the extent that the continuity of tubular structure is disrupted. The lesions may affect any segment of the tubules, appearing at irregular intervals along with normal segments throughout the kidney. Reepithelialization in the areas with intact basement membrane heals tubular lesions. Such healing is unable to take place in areas in which the basement membrane has been disrupted; connective tissue grows through the ruptured membrane, thus preventing reestablishment of tubular integrity. Individual cells within the nephron, but not the entire nephron, are capable of regeneration. Clinical Course. The clinical course of the child with AKI is variable and depends on the cause. In reversible AKI there is a period of severe oliguria, or a low-output phase, followed by an abrupt onset of diuresis, or a high-output phase; this phase is followed by a gradual return to, or toward, normal urine volumes. The length of the oliguric phase in older children and adolescents is 10 to 14 days but is highly variable at all ages depending on the cause of the AKI. The onset of the diuretic phase appears unexpectedly, and over several days it proceeds in stepwise fashion from very low to above-normal urine volumes. During the oliguric phase, manifestations of uremia are present but may also be accompanied by other clinical disorders that make assessment difficult, such as infection, anoxia, and shock. Clinical Manifestations In many instances of AKI the infant or child is already critically ill with the precipitating disorder, and the explanation for development of oliguria may or may not be readily apparent. The underlying illness often overshadows the renal failure and often assumes the priority of care (e.g., the patient who is in shock from endotoxemia, the infant who is severely dehydrated from gastroenteritis, or a child who is subject to seizures as a result of hypertensive encephalopathy associated with AGN). The prime manifestation of AKI is oliguria, generally a urinary output of less than 1 ml/kg/hr. Anuria (no urinary output in 24 hours) is uncommon, except in obstructive disorders. Other symptoms related to AKI include edema, drowsiness, circulatory congestion, and cardiac arrhythmia from hyperkalemia. Seizures may be caused by hyponatremia or hypocalcemia and tachypnea from metabolic acidosis. With continued oliguria, biochemical abnormalities can develop rapidly, and circulatory and central nervous system manifestations appear. Diagnostic Evaluation When a previously well child develops AKI without obvious cause, a careful history is obtained to reveal symptoms that may be related to glomerulonephritis; obstructive uropathy; or exposure to nephrotoxic chemicals, such as ingestion of heavy metals or inhalation of carbon tetrachloride or other organic solvents or drugs (e.g., methicillin, sulfonamides, NSAIDs, neomycin, polymyxin, and kanamycin). Laboratory data reflect the kidney dysfunction: hyperkalemia, hyponatremia, metabolic acidosis, hypocalcemia, anemia, or azotemia (Table 24.7). TABLE 24.7 Laboratory Findings Associated With Acute Renal Failure Clinical Problem Mechanism Clinical Considerations Azotemia Ongoing protein catabolism Lower rate of production in neonates and persons with depleted protein stores Elevated blood urea nitrogen levels Significantly decreased excretion Increased in situations involving large amounts of necrotic tissue or extravasated blood Elevated plasma creatinine levels Continued production Significantly decreased excretion Production less affected by other factors More sensitive measure of intensity of azotemia Low in neonate because of small muscle mass relative to size Metabolic acidosis Continued endogenous acid production Significantly decreased excretion Depletion of extracellular and intracellular fluid buffers Compensatory hyperventilation Opisthonos Major threat to life Hyponatremia Dilution of extracellular fluid Decreased excretion of water May develop cerebral signs Hyperkalemia Ongoing protein catabolism Decreased excretion compounded by metabolic acidosis Most important electrolyte to be considered in acute renal failure May contribute to cardiac arrhythmia With electrocardiogram changes, major threat to life Loss may be from gastrointestinal tract Hypocalcemia Associated with metabolic acidosis and hyperphosphatemia During alkali therapy, may cause tetany Therapeutic Management The most effective management of AKI is prevention. The development of AKI is a known risk in certain situations. This should be anticipated and recognized, and adequate therapy should be implemented (e.g., fluid therapy for children with hypovolemia in conditions such as dehydration, burns, and hemorrhage). Nephrotoxic drugs should be used with caution or avoided in children with renal disease, and all personnel should be knowledgeable about precautions related to their administration. For example, a generous fluid intake is needed for children receiving antimetabolite drugs and after radiotherapy. The treatment of AKI is directed toward treatment of the underlying cause, management of the complications of renal failure, and provision of supportive therapy within the constraints imposed by the renal failure. Treatment of poor perfusion resulting from dehydration consists of volume restoration as described in the treatment of dehydration. (See Chapter 23.) If oliguria persists after restoration of fluid volume or if the renal failure is caused by intrinsic renal damage, the physiologic and biochemical abnormalities that have resulted from kidney dysfunction must be corrected or controlled. Central venous pressure monitoring is usually implemented. Initially a catheter is inserted to rule out urine retention, to collect available urine for electrolytes and analysis, and to monitor the results of diuretic administration. The catheter may or may not be removed. Some clinicians believe that it serves little purpose during the oliguric phase and predisposes the patient to bladder infections. Others maintain a catheter for hourly urine measurements. The use of mannitol, loop diuretics such as furosemide and other medications in the prevention and treatment of AKI have been studied. The 2012 KDIGO Guidelines on Acute Kidney Injury state that the use of mannitol in the prevention of AKI is not scientifically justified due to inadequate studies. They do not recommend the use of diuretics to prevent or treat AKI except as an aid in the management of volume overload. Fluid and Calories. The amount of exogenous water provided should not exceed the amount needed to maintain zero water balance. It is calculated on the basis of estimated endogenous water formation and losses from sensible (primarily gastrointestinal) and insensible sources. No allotment is calculated for urine as long as oliguria persists. The child with AKI has a tendency to develop water intoxication and hyponatremia, both of which make it difficult to provide calories in sufficient amounts to meet the child's needs and reduce tissue catabolism, metabolic acidosis, hyperkalemia, and uremia. If the child is able to tolerate oral foods, concentrated food sources that are high in carbohydrates and fat but low in protein, potassium, and sodium may be provided. However, many children have functional disturbances of the gastrointestinal tract, such as nausea and vomiting. Therefore the IV route is generally preferred, and nourishment usually consists of essential amino acids or a combination of essential and nonessential amino acids administered by the central venous route. Control of water balance in these patients requires careful monitoring of feedback information, such as accurate intake and output, body weight, and electrolyte measurements. In general, during the oliguric phase, no sodium, chloride, or potassium is given unless there are other large, ongoing losses. Regular measurement of plasma electrolytes, pH, BUN, and creatinine levels is required to assess the adequacy of fluid therapy and to anticipate complications that require specific treatment. Hyperkalemia. An elevated serum potassium level is the most immediate threat to the life of the child with AKI. Potassium ions are not being excreted, while at the same time the release of potassium from cells is accelerated by acidosis, stress, and tissue breakdown in cases associated with internal bleeding or trauma. Because cardiac arrhythmia and cardiac arrest may result, electrocardiograms (ECGs) and serum potassium ion levels are monitored regularly. Hyperkalemia can be minimized and sometimes avoided by eliminating potassium from all food and fluids, by reducing tissue catabolism, and by correcting acidosis. Nursing Alert Any of the following signs of hyperkalemia constitute an emergency and should be reported immediately: • Serum potassium concentrations in excess of 7 mEq/L • Presence of ECG abnormalities, such as loss of P wave, prolonged RS complex, depressed ST segment, tall and tented T waves, bradycardia, or heart block Several measures are available to reduce the serum potassium concentration, and the priority of implementation is usually based on the rapidity with which the measures are effective. Temporary measures that produce a rapid but transient effect are as follows: • Calcium gluconate administered intravenously over 2 to 4 minutes with continuous ECG monitoring, exerts a protective effect on cardiac conduction. • Sodium bicarbonate administered intravenously over 30 to 60 minutes, elevates the serum pH to cause a transient shift of extracellular fluid potassium into the intracellular fluid. However, there is a risk of hypocalcemia, tetany, and fluid overload. • Glucose and insulin administered intravenously, accelerate glycogen synthesis, causing glucose and potassium to move into the cells. Insulin facilitates the entry of glucose into cells. These effects produce only transient protection by redistributing existing potassium stores; they do not remove potassium from the body. However, they provide relief while more definitive but slower-acting measures are being implemented. Potassium can be removed by either of two methods: 1. Administration of a cation exchange resin such as sodium polystyrene sulfonate (Kayexalate), 1 g/kg, administered orally or rectally, to bind potassium and remove it from the body. This requires time to be effective, and a sodium ion is exchanged for each potassium ion. This increased sodium concentration adds to the body fluids, which may contribute to fluid overload, hypertension, and cardiac failure. 2. Dialysis or continuous hemofiltration (see p. 810). Hemodialysis is efficient but requires specialized facilities. PD is simpler and can be carried out in almost any hospital setting. Indications for dialysis in AKI are continued oliguria associated with any of the following: • Severe, persistent acidosis • Inability to reduce serum potassium levels to a safe range with other methods • Clinical uremic syndrome consisting of nausea and vomiting, drowsiness, and progression to coma • Circulatory overload, hypertension, and evidence of cardiac failure The optimal timing for initiation of renal replacement therapy is controversial. One strategy is to institute renal replacement therapy within hours of the diagnosis of severe AKI being made, regardless of other symptoms. Another strategy is to delay renal replacement therapy until any of the previously listed symptoms presents. Differences in mortality have not been demonstrated between the two approaches (Gaudry, Hajage, Schortgen, et al., 2016). Hypertension. Hypertension is a common and serious complication of AKI, and blood pressure determinations are taken at least every 4 to 6 hours to detect it early. The most common cause of hypertension in AKI is overexpansion of the extracellular fluid and plasma volume, together with activation of the renin-angiotensin system. The goal of therapy is to prevent hypertensive encephalopathy and avoid overtaxing the cardiovascular system. When there is a threat of encephalopathy, labetalol (a beta and alpha blocker) may be administered intravenously as bolus infusions or a continuous drip. Sodium nitroprusside may be given but requires close monitoring. For less urgent situations, hydralazine, clonidine, or verapamil may be given intravenously. Oral drugs used for acute hypertension include nifedipine, captopril, minoxidil, hydralazine, propranolol, or furosemide. Other Complications. Other complications that may occur with AKI are anemia, seizures and coma, cardiac failure, and pulmonary edema. Anemia is commonly associated with AKI, but transfusion is not recommended unless the hemoglobin level drops below 6 g/dl. Transfusions consist of fresh, packed red blood cells given slowly to reduce the likelihood of increasing blood volume, hypertension, and hyperkalemia. Seizures occur often when renal failure progresses to uremia and are also related to hypertension, hyponatremia, and hypocalcemia. Treatment is directed toward the specific cause when known. More obscure causes are managed with antiepileptic drugs. Cardiac failure with pulmonary edema is almost always associated with hypervolemia. Treatment is directed toward reduction of fluid volume, with water and sodium restriction and administration of diuretics. Digitalis is ineffective and can be hazardous. Diuretic, or High-Output, Phase. When the output begins to increase, either spontaneously or in response to diuretic therapy, the nurse should monitor the intake of fluid, potassium, and sodium, and provide adequate replacement to prevent depletion and its consequences. In some cases the high-output phase is mild and lasts only a few days; in others enormous amounts of electrolyte-rich urine are passed. Prognosis. The prognosis of AKI depends largely on the nature and severity of the causative factor or precipitating event and the promptness and competence of management. The mortality rate is less than 20%. The outcome is least favorable in children with rapidly progressive nephritis and cortical necrosis. Children in whom AKI is a result of HUS or AGN may recover completely, but residual renal impairment or hypertension is more often the rule. Complete recovery is usually expected in children whose renal failure is a result of dehydration, nephrotoxins, or ischemia. AKI after cardiac surgery has a less favorable prognosis. It is often impossible to assess the extent of recovery for several months. (See Quality Patient Outcomes box.) Quality Patient Outcomes Acute Kidney Injury • Underlying cause of acute kidney injury identified and treated • Water balance maintained • Hypertension controlled • Electrolyte balance maintained • Diet maintains calories while minimizing tissue catabolism, metabolic acidosis, hyperkalemia, and uremia Nursing Care Management Nursing care of the infant or child with AKI involves addressing the underlying cause plus carefully observing and managing the renal status. The major goal is reestablishment of renal function (with emphasis on providing an adequate caloric intake to minimize reduction of protein stores); prevention of complications; and monitoring of fluid balance, laboratory data, and physical manifestations. The probability of dialysis or continuous hemofiltration is high, and the nurse must anticipate the availability of the necessary equipment. Because the child requires intensive observation and often specialized equipment, the usual disposition is admission to an intensive care unit where equipment and trained personnel are available. The major nursing tasks in the care of the infant or child with AKI are monitoring and assessing fluid and electrolyte balance. Limiting fluid intake requires ingenuity on the part of caregivers to cope with the child who is thirsty. One strategy involves rationing the daily intake with small amounts of fluid served in containers that give the impression of larger volumes. Older children who understand the rationale of fluid limits can help determine how their daily ration should be distributed. Meeting nutritional needs is sometimes a problem because the child may be nauseated and because getting the child to eat concentrated foods without fluids may be difficult. When nourishment is provided by the IV route, careful monitoring is essential to prevent fluid overload. This can become a major challenge in the face of nutritional requirements and administration of IV medications. The IV drugs being used may be nephrotoxic, which can require a specified volume of solution for delivery. In some instances blood products must also be delivered. Preventing fluid overload while delivering medications and calories requires concerted collaboration. In addition, nursing measures such as maintaining an optimum thermal environment, reducing any elevation of body temperature, and reducing restlessness and anxiety are used to decrease the rate of tissue catabolism. The nurse must be continually alert for behavior changes that indicate the onset of complications. Infection from reduced resistance, anemia, and general morbidity is a constant threat. Fluid overload and electrolyte disturbances can precipitate cardiovascular complications such as hypertension and cardiac failure. Fluid and electrolyte imbalances, acidosis, and accumulation of nitrogenous waste products can produce neurologic involvement manifested by coma, seizures, or alterations in sensorium. Although children with AKI are usually quite ill and voluntarily diminish their activity, infants may become restless and irritable, and children are often anxious and frightened. Frequent, painful, and stress-producing treatments and tests must be performed. A supportive, empathetic nurse can provide comfort and stability in a threatening and unnatural environment. Family Support. Providing support and reassurance to parents is among the major nursing responsibilities. The seriousness and emergency nature of AKI are stressful to parents, and most feel some degree of guilt regarding the child's condition, especially when the illness is the result of ingestion of a toxic substance, dehydration, or a genetic disease. They need reassurance and an empathetic listener. They also need to be kept informed of the child's progress and provided explanations regarding the therapeutic regimen. The equipment and the child's behavior are sometimes frightening and anxiety provoking. Nurses can do much to help parents comprehend and deal with the stresses of the situation.
ChatGPT said:
ChatGPT
Acute Kidney Injury (AKI) Overview
Definition: Acute Kidney Injury (AKI) occurs when the kidneys suddenly lose their ability to regulate urine volume and composition in response to food and fluid intake. Key features include oligoanuria (reduced urine output), azotemia (elevated nitrogenous waste in the blood), acidosis, and electrolyte imbalances. While not common in children, early recognition and treatment are crucial for favorable outcomes.
Etiology
AKI can arise from various clinical conditions, often classified into three categories:
Prerenal Causes:
Description: Related to reduced renal perfusion in a structurally normal kidney.
Common Causes:
Dehydration (from diarrhea or vomiting)
Surgical shock
Trauma (including burns)
Mechanism: Decreased renal perfusion leads to reduced glomerular filtration rate (GFR) and compensatory hormonal responses, potentially causing cortical or tubular necrosis if prolonged.
Intrinsic Renal Causes:
Description: Involves direct damage to the kidney tissues (glomeruli, tubules, or vasculature).
Common Causes:
Glomerular diseases (e.g., glomerulonephritis)
Tubular necrosis (ischemia or nephrotoxins)
Mechanism: Varies depending on the type and extent of damage, leading to varying degrees of renal dysfunction.
Postrenal Causes:
Description: Resulting from obstruction in the urinary tract.
Common Causes: Rare in children but may occur within the first year of life.
Mechanism: Relief of obstruction may restore function; duration of obstruction affects recovery.
Pathophysiology
Glomerular Filtration Rate (GFR): Severely reduced, leading to elevated Blood Urea Nitrogen (BUN) and altered sodium reabsorption.
Hormonal Responses: Increased renin secretion causes afferent arteriolar vasoconstriction, further reducing GFR.
Necrosis Types:
Cortical Necrosis: Severe ischemia or infections; most common in neonates.
Tubular Necrosis: Ischemia and nephrotoxic injuries, resulting in tubular epithelial cell death and possible incomplete recovery.
Clinical Course
Phases of AKI:
Oliguric Phase: Low urine output (<1 ml/kg/hr) with possible anuria.
Diuretic Phase: Abrupt increase in urine output, potentially leading to electrolyte imbalances.
Duration of the oliguric phase can vary significantly based on underlying causes.
Clinical Manifestations
Key Symptoms:
Oliguria or anuria
Edema
Drowsiness
Hypertension
Cardiac arrhythmias due to hyperkalemia
Neurologic symptoms (seizures, altered mental status)
Diagnostic Evaluation
Key Laboratory Findings:
Azotemia: Elevated BUN and creatinine due to decreased excretion.
Electrolyte Imbalances: Hyperkalemia, hyponatremia, metabolic acidosis.
Anemia: Due to chronic illness or renal dysfunction.
Therapeutic Management
Prevention: Recognize at-risk situations and implement fluid therapy and nephrotoxin precautions.
Fluid and Electrolyte Management:
Restore volume for prerenal causes.
Monitor and restrict electrolytes based on levels and patient needs.
Management of Hyperkalemia:
Monitor serum potassium levels and ECG changes.
Temporary interventions: Calcium gluconate, sodium bicarbonate, insulin and glucose.
Definitive treatment: Sodium polystyrene sulfonate (Kayexalate) or dialysis.
Blood Pressure Control:
Monitor blood pressure every 4–6 hours.
Use antihypertensive medications (e.g., labetalol, sodium nitroprusside) as needed.
Nutritional Support:
Provide high-calorie, low-protein, low-potassium diets.
IV nutritional support if oral intake is insufficient.
Monitoring and Supportive Care:
Intensive monitoring of fluid balance, vital signs, and laboratory values.
Family support and education regarding the child's condition.
Prognosis
Outcomes: Dependent on the cause, severity, and timeliness of management. Mortality rates are generally less than 20%, with favorable outcomes expected in cases of dehydration or nephrotoxins, while HUS or rapidly progressive nephritis may lead to long-term complications.
Nursing Care Management
Focus on monitoring fluid and electrolyte balance, preventing complications, and providing family support.
Prepare for potential dialysis needs and ensure comprehensive care coordination in intensive settings.
Offer empathetic support to both child and family, addressing emotional and informational needs during treatment.
4o mini
You said:
Laboratory Findings Associated With Acute Renal Failure Clinical Problem Mechanism Clinical Considerations Azotemia Ongoing protein catabolism Lower rate of production in neonates and persons with depleted protein stores Elevated blood urea nitrogen levels Significantly decreased excretion Increased in situations involving large amounts of necrotic tissue or extravasated blood Elevated plasma creatinine levels Continued production Significantly decreased excretion Production less affected by other factors More sensitive measure of intensity of azotemia Low in neonate because of small muscle mass relative to size Metabolic acidosis Continued endogenous acid production Significantly decreased excretion Depletion of extracellular and intracellular fluid buffers Compensatory hyperventilation Opisthonos Major threat to life Hyponatremia Dilution of extracellular fluid Decreased excretion of water May develop cerebral signs Hyperkalemia Ongoing protein catabolism Decreased excretion compounded by metabolic acidosis Most important electrolyte to be considered in acute renal failure May contribute to cardiac arrhythmia With electrocardiogram changes, major threat to life Loss may be from gastrointestinal tract Hypocalcemia Associated with metabolic acidosis and hyperphosphatemia During alkali therapy, may cause tetany
ChatGPT said:
ChatGPT
Laboratory Findings Associated With Acute Renal Failure
Clinical ProblemMechanismClinical Considerations | ||
Azotemia | Ongoing protein catabolism | Lower rate of production in neonates and individuals with depleted protein stores. |
Elevated blood urea nitrogen levels | Significantly decreased excretion; increased in situations involving large amounts of necrotic tissue or extravasated blood. | |
Elevated plasma creatinine levels | Continued production; significantly decreased excretion; production less affected by other factors. More sensitive measure of intensity of azotemia; levels are low in neonates due to small muscle mass relative to size. | |
Metabolic acidosis | Continued endogenous acid production | Significantly decreased excretion; depletion of extracellular and intracellular fluid buffers; compensatory hyperventilation; opisthonos; major threat to life. |
Hyponatremia | Dilution of extracellular fluid | Decreased excretion of water; may develop cerebral signs. |
Hyperkalemia | Ongoing protein catabolism | Decreased excretion compounded by metabolic acidosis; most important electrolyte to consider in acute renal failure; may contribute to cardiac arrhythmia; with electrocardiogram changes, poses a major threat to life. Loss may occur from the gastrointestinal tract. |
Hypocalcemia | Associated with metabolic acidosis and hyperphosphatemia | During alkali therapy, may cause tetany. |
This table summarizes the key laboratory findings that indicate the presence of acute renal failure, including the underlying mechanisms and clinical considerations for each abnormality. Monitoring these findings is crucial for the management and treatment of patients with acute kidney injury.
4o mini
You said:
Discuss laboratory findings associated with acute renal failure.
ChatGPT said:
ChatGPT
Acute renal failure (ARF), also known as acute kidney injury (AKI), is characterized by a sudden decline in kidney function, which leads to the accumulation of waste products in the blood and an imbalance of electrolytes and fluids. Various laboratory findings are associated with ARF, reflecting the underlying pathophysiology and guiding management. Here’s a discussion of the key laboratory findings:
1. Azotemia
Definition: Azotemia refers to an increase in blood urea nitrogen (BUN) and creatinine levels due to the accumulation of nitrogenous waste products in the bloodstream.
Mechanism: In ARF, there is a combination of ongoing protein catabolism and significantly decreased renal excretion.
Clinical Considerations:
BUN levels can rise significantly in conditions with extensive tissue necrosis (e.g., rhabdomyolysis) or significant blood loss.
Creatinine levels are a more sensitive marker for kidney function, as their production is less influenced by diet and muscle mass. In neonates, creatinine levels may be low due to a smaller muscle mass.
2. Electrolyte Abnormalities
Hyponatremia:
Mechanism: Often occurs due to the dilution of extracellular fluid or impaired renal excretion of water.
Clinical Considerations: Can lead to neurological symptoms, including seizures or confusion, if severe.
Hyperkalemia:
Mechanism: Results from ongoing protein catabolism and decreased renal excretion, particularly in the context of metabolic acidosis.
Clinical Considerations: Hyperkalemia is particularly dangerous as it can cause life-threatening cardiac arrhythmias. Electrocardiogram (ECG) changes (e.g., peaked T-waves) are critical indicators.
Hypocalcemia:
Mechanism: Often associated with metabolic acidosis and hyperphosphatemia, where phosphate levels rise and bind calcium, leading to decreased serum calcium.
Clinical Considerations: Hypocalcemia can lead to tetany, especially during alkalizing treatment for acidosis.
3. Metabolic Acidosis
Mechanism: ARF leads to decreased excretion of acids and ongoing endogenous acid production, which results in a lower blood pH.
Clinical Considerations: Patients may exhibit compensatory mechanisms, such as hyperventilation. Severe acidosis can result in muscle spasms (opisthonos) and represents a significant threat to life.
4. Other Laboratory Findings
Urinalysis:
Changes in urine output (oliguria or anuria) are common. Urinalysis may show casts, protein, or blood depending on the underlying cause of renal failure.
Imaging Studies:
While not laboratory tests per se, imaging studies such as ultrasound can help assess for obstructions or abnormalities in renal structure.
Summary
The laboratory findings associated with acute renal failure are critical for diagnosing and managing the condition. Elevated levels of BUN and creatinine indicate decreased kidney function, while electrolyte imbalances such as hyperkalemia and hyponatremia highlight the acute nature of renal impairment and its systemic effects. Monitoring these parameters allows healthcare providers to guide treatment effectively and mitigate potential complications.
4o mini
You said:
Compare and contrast the pathophysiology, clinical manifestations, and nursing management of children with acute renal failure and with chronic renal failure (CRF). Chronic Kidney Disease The kidneys are able to maintain the chemical composition of fluids within normal limits until more than 50% of functional renal capacity is destroyed by disease or injury. Chronic kidney disease (CKD) occurs when the diseased kidneys can no longer maintain the normal chemical structure of body fluids under normal conditions. Progressive deterioration over months or years produces a variety of clinical and biochemical disturbances that conclude in the clinical syndrome known as uremia. The final stage of CKD, ESRD, is irreversible. Treatment with dialysis or transplantation is required when the glomerular filtration rate decreases below 10% to 15% of normal. The pattern of renal dysfunction is remarkably uniform no matter what disease process initiates the advanced disease. Etiology A variety of diseases and disorders can result in CKD. The most common causes of CKD before age 5 years are congenital renal and urinary tract malformations (particularly renal hypoplasia and dysplasia and obstructive uropathy) and VUR. Glomerular and hereditary renal diseases predominate in children 5 to 15 years of age. The glomerular diseases that most commonly lead to CKD are chronic pyelonephritis, CGN, and glomerulonephropathy associated with systemic diseases such as anaphylactoid purpura and lupus erythematosus. Hereditary nephritis, congenital nephrotic syndrome, Alport syndrome, polycystic kidney, and several other hereditary disorders result in renal failure in childhood. Renal vascular disorders such as HUS, vascular thrombosis, or cortical necrosis are less common causes. Pathophysiology Early in the course of progressive nephron destruction, the child remains asymptomatic with only minimum biochemical abnormalities. Unless its presence is detected in the process of routine assessment, signs and symptoms that indicate advanced renal damage often emerge only late in the course of the disease. Midway in the disease process, as increasing numbers of nephrons are totally destroyed, and most others are damaged to varying degrees; the few that remain intact are hypertrophied but functional. These few normal nephrons are able to make sufficient adjustments to stresses to maintain reasonable degrees of fluid and electrolyte balance. Definitive biochemical examination at this time reveals restricted tolerance to excesses or restrictions. As the disease progresses to the end stage because of severe reduction in the number of functioning nephrons, the kidneys are no longer able to maintain fluid and electrolyte balance, and the features of uremia appear. The following sections briefly summarize the pathophysiology of specific biochemical abnormalities. Retention of Waste Products. Serum creatinine and BUN levels are utilized to evaluate renal function. Creatinine is a waste product of muscle catabolism. Because muscle mass is relatively stable, creatinine production is also stable. Most creatinine is filtered out by the kidneys and expelled in the urine. BUN (a by-product of protein breakdown) levels also increase as kidney function declines. BUN is a less precise marker than creatinine as is not produced at a stable rate and can be influenced by protein intake, hydration status and other factors (Lopez-Giacoman & Madero, 2015). Water and Sodium Retention. The damaged kidneys are able to maintain sodium and water balance under normal circumstances, although the few remaining functional nephrons are required to increase their rate of filtration and reabsorption in proportion to their numbers. The limitations of this capacity become apparent under stress. The nature of abnormalities in adjustment depends on the underlying renal disease. Infants and small children with kidney dysplasia or urinary obstructive disease tend to excrete large volumes of dilute urine low in sodium content. Children with glomerular disease tend to retain both sodium and water as a result of a greater reduction of glomerular filtration than of tubular reabsorption. Children with defective sodium reabsorption from tubular disease tend to lose sodium, with a corresponding osmotic water loss. Consequently, sodium excesses may cause edema and hypertension, whereas sodium deprivation can result in hypovolemia and circulatory failure. Only in ESRD is markedly reduced glomerular filtration inadequate to handle normal amounts of sodium and water. Retention of these substances leads to edema and vascular congestion. Hyperkalemia. Dangerous hyperkalemia is uncommon in CKD until the end stage. However, the kidneys are unable to adjust readily to increased ingestion of potassium, and they require a longer period to rid the body of this excess. Acidosis. A sustained metabolic acidosis is characteristic of CKD; it results from the damaged kidney's inability to excrete a normal load of metabolic acids generated by normal metabolic processes. There is reduced capacity of the distal tubules to produce ammonia and impaired reabsorption of bicarbonate. Despite continuous hydrogen ion retention and bicarbonate loss, the plasma pH is maintained at a level compatible with life by other buffering mechanisms, particularly the bone salt (see the following sections). Calcium and Phosphorus Disturbances. Calcium and phosphorus homeostasis are affected by CKD. Profound and complex disturbances in the metabolism of these substances result in significant bone demineralization and impaired growth. This appears to be related to several factors (Box 24.8). These complex disturbances in calcium, phosphorus, and bone metabolism produce growth arrest or delay; bone pain; and deformities known as renal osteodystrophy, sometimes called renal rickets, because the disorganization of bone growth and demineralization are similar to that caused by vitamin D–resistant rickets. Box 24.8 Factors Related to Bone Demineralization in Chronic Renal Failure • In a state of acidosis there is dissolution of the alkaline salts of bone, which serve as buffers, and the release of phosphorus and calcium into the bloodstream. • Reduced glomerular filtration and excretion of inorganic phosphate lead to an elevation of plasma phosphate with a concomitant decrease in serum calcium. • Decreased serum calcium concentration stimulates the secretion of parathyroid hormone, which results in reabsorption of calcium from bones. Under normal circumstances parathyroid hormone inhibits the tubular reabsorption of phosphates. • Diseased kidneys are unable to complete the synthesis of vitamin D to its most active form, 1,25-dihydroxycholecalciferol, which is necessary for the absorption of calcium from the gastrointestinal tract and deposition of calcium in bone. This acquired resistance to vitamin D decreases calcium absorption, permits retention of phosphorus, and contributes to secondary hyperparathyroidism. Anemia. A consistent feature of CKD is anemia, which appears to result from several factors (Box 24.9). Box 24.9 Causes of Anemia in Chronic Renal Failure • Shortened life span of red blood cells caused by some extracorpuscular factor associated with the uremic state • Impaired red blood cell production resulting from decreased production of erythropoietin • Blood loss related to increased tendency to bleed, associated with a prolonged bleeding time, probably related to impaired platelet function and laboratory blood samples • Hyperparathyroidism • Hypersplenism, which may be related to silicone deposition (from dialysis blood lines) and granuloma formation in the spleen • Diseases related to hemolytic anemia, such as systemic lupus erythematosus and sickle cell disease Growth Disturbance. One of the most striking effects of CKD in childhood, and one that can have profound psychologic and social consequences for the developing child, is delayed growth. The cause is poorly understood but may be related to nutritional and biochemical factors (Box 24.10). Box 24.10 Probable Causes of Growth Failure in Chronic Renal Failure • Renal osteodystrophy • Poor nutrition associated with dietary restrictions (especially protein) and loss of appetite • Biochemical abnormalities associated with renal failure, such as sustained acidosis or renal sodium wasting • Hypertension • Corticosteroid treatment • Tissue resistance to growth hormone • Trace mineral and vitamin deficiencies Sexual maturation may be delayed or may not occur in children with CKD, and secondary amenorrhea commonly develops in girls past puberty. CKD can also cause sexual dysfunction by creating imbalances in gonadal hormone levels. Decreased testosterone levels impair spermatogenesis in males; decreased estrogen, luteinizing hormone, and progesterone cause anovulation and menstrual irregularities (usually amenorrhea) in females. Autonomic neuropathy and anemia are also factors that can alter sexual function. Other Disturbances. Children with CKD are more susceptible to infection, especially pneumonia, UTI, and septicemia, although the reason for this is not entirely clear. Hyperventilation, a manifestation of the respiratory compensatory mechanism for metabolic acidosis, and pulmonary edema may contribute to upper respiratory tract infection. These children become extraordinarily sensitive to changes in vascular volume that may cause, in addition to pulmonary overload, cerebral symptoms and circulatory manifestations such as hypertension and cardiac failure. Numerous neurologic manifestations appear with advanced renal failure, although no specific toxin or biochemical defect has been identified. However, disturbances in enzyme function, disturbances in water and electrolyte balance, altered calcium ion concentration, hypertension, and accumulation of various “uremic toxins” have been implicated. Clinical Manifestations The first symptom of CKD may be loss of normal energy and increased fatigue on exertion. For example, the child may prefer quiet, passive activities rather than participation in more active games and outdoor play. The child is usually somewhat pale, but the change is often so subtle that it may not be evident to parents or others. Blood pressure is sometimes elevated. Growth is affected early in the development of CKD, and falling behind on the growth chart is often the first measurable sign. Other manifestations may appear as the disease progresses. The child does not eat as well (especially breakfast), shows less interest in normal activities such as schoolwork or play, and has a decreased or increased urinary output and a compensatory intake of fluid. For example, a child who has achieved bladder control may wet the bed at night. Pallor becomes more evident as the skin develops a characteristic sallow, muddy appearance as a result of anemia and deposition of urochrome pigment in the skin. The child may complain of headache, muscle cramps, and nausea. Other signs and symptoms include weight loss, facial puffiness, malaise, bone or joint pain, growth retardation, dryness or itching of the skin, bruised skin, and sometimes sensory or motor loss. Amenorrhea is common in adolescent girls. Therapy is generally initiated before the appearance of the uremic symptoms, although on some occasions the symptoms may be observed. Manifestations of untreated uremia reflect the progressive nature of the homeostatic disturbances and general toxicity. Gastrointestinal symptoms include loss of appetite, nausea, and vomiting. Bleeding tendencies are apparent in bruises, bloody diarrheal stools, stomatitis, and bleeding from the lips and mouth. Intractable itching occurs, probably related to a number of factors, including dry skin and hyperparathyroidism (Wojtowicz-Prus, Kiliś-Pstrusińska, Reich, et al., 2016). Deposits of urea crystals may appear on the skin as uremic frost but are seldom seen because of the availability of dialysis and transplantation. There may be an unpleasant uremic odor to the breath. Respirations become deeper as a result of metabolic acidosis, and circulatory overload is manifested by hypertension, congestive heart failure, and pulmonary edema. Progressive confusion, dulling of the sensorium, and ultimately coma are signs of neurologic involvement. Other signs may include tremors, muscular twitching, and seizures. Diagnostic Evaluation The diagnosis of CKD is usually suspected on the basis of any of a number of clinical manifestations, a history of prior renal disease, or biochemical findings. The onset is usually gradual, and the initial signs and symptoms are vague and nonspecific. Laboratory and other diagnostic tools and tests are of value in assessing the extent of renal damage, biochemical disturbances, and related physical dysfunction. Often, they can help establish the nature of the underlying disease and differentiate between other disease processes and the pathologic consequences of renal dysfunction. Therapeutic Management Classification of CKD as stage 1 (GFR ≥90) through stage 5 (GFR <15 or dialysis) helps with evaluation and management decisions through the use of medical protocols such as the National Kidney Foundation Kidney Disease Outcomes Quality Initiative evidence-based clinical practice guidelines (http://www.kidney.org/professionals/KDOQI). The goals of management are to maximize effective renal function, maintain body fluid and electrolyte balance within acceptable limits, treat systemic complications, and promote as active and normal a life as possible for the child for as long as possible. This becomes increasingly difficult as the disease progresses toward end stage. Therapeutic measures designed to relieve one manifestation may negatively affect another. For example, antihypertensive agents may further impair renal function. Activity. Allow children unrestricted activity and to set their own limits regarding rest and extent of exertion. Encourage them to attend school. If the effort is too great, home tutoring can be arranged. Diet. Regulation of diet has been seen as the most effective means, short of dialysis, for reducing the quantity of materials that require renal excretion. The goal of the diet in renal failure is to provide sufficient calories and protein for growth while minimizing the excretory demands made on the kidney, to limit metabolic bone disease (osteodystrophy), and to minimize fluid and electrolyte disturbances. Dietary protein intake is limited to the recommended dietary allowance (RDA) for the child's age. Restriction of protein intake below the RDA is believed to negatively affect growth and neurodevelopment. Dietary phosphorus may need to be restricted. Remember that any attempt to restrict dietary intake in children potentially restricts caloric intake and can limit growth. Protein in the diet should include foods of high biologic value. When given with meals, substances that bind phosphorus in the intestines prevent its absorption and allow a more liberal intake of phosphorus-containing protein. Sodium and water are not usually limited unless there is evidence of edema or hypertension. Potassium is not restricted as long as creatinine clearance remains at acceptable limits (30 to 35 ml/min). However, restrictions are instituted for patients with oliguria or anuria. Restrictions of any or all of these minerals may be imposed in later stages or at any time in which factors cause abnormal serum concentrations. Because of modified dietary intake, altered metabolism, and poor appetite, some dietary supplementation is usually needed. Because fat-soluble vitamins can accumulate in patients with CKD, vitamins A, E, and K are not supplemented beyond normal dietary intake. Active and/or inactive forms of vitamin D are prescribed, and water-soluble vitamin supplementation may be required if the diet is inadequate. Other dietary needs are discussed in relation to osteodystrophy and anemia. Dietary management of the child with renal failure is a difficult and complex problem that necessitates collaboration with a registered dietitian who is knowledgeable about pediatric nutrition and the impact of renal failure. Osteodystrophy. Measures directed at prevention or correction of the calcium/phosphorus imbalance are reduction of dietary phosphorus, administration of a phosphorus-binding agent, provision of supplemental calcium, control of acidosis, and administration of an active and/or inactive form of vitamin D. The reduction of protein and milk intake can control dietary phosphorus. Oral administration of phosphorus-binding agents, which combine with the phosphorus to decrease gastrointestinal absorption and thus the serum levels of phosphate, can further reduce phosphorus levels. Calcium carbonate preparations can be used as phosphorus binders. These medications act as phosphate binders, calcium supplements, and alkalizing agents. Calcium carbonate preparations can be given with meals to bind phosphorus if the child is hyperphosphatemic or mildly hypocalcemic. If given 1 to 2 hours after meals, they act as calcium supplements for children with stable phosphorus but low calcium levels. Calcium acetate can also be used. Newer, iron-based binders can be used when there is concern for hypercalcemia (Stormont, McCoy, Bashir, et al., 2016). Aluminum hydroxide gels are effective phosphorus binders but have been shown to cause aluminum loading when used in children with renal failure. Aluminum intoxication leads to altered sensorium, an inability to talk, ataxia, seizures, and severe bone disease. Secondary hyperparathyroidism (evidenced by an elevated intact PTH level), in a child with normal phosphate, calcium and 25OH vitamin D levels requires treatment with an active form of vitamin D. Oral medications such as calcitriol (Rocaltrol) increase absorption of calcium through the gastrointestinal tract. The serum calcium level is monitored frequently during periods when the drugs are being initiated or changed to detect hypercalcemia. Intact parathyroid hormone levels are measured every 2 to 3 months with target levels based on the stage of CKD. Osseous deformities that result from renal osteodystrophy, especially those related to ambulation, are troublesome and require correction if they occur. Careful attention to the management of osteodystrophy and bone growth can prevent deformities in some children. Acidosis. Pharmacologic treatment of acidosis is initiated early in children who have chronic renal insufficiency. In addition to reducing the formation of metabolic acids by avoiding excessive dietary protein intake, alkalizing agents such as sodium bicarbonate or a combination of sodium and potassium citrate (Bicitra, Polycitra, or Shohl solution) alleviate acidosis. Correction of acidosis is best attempted after calcium levels are elevated because rapid correction may precipitate tetany in a hypocalcemic child. Anemia. Because the anemia associated with renal failure is related to decreased production of erythropoietin, it usually cannot be successfully managed with hematinic agents. Provide sufficient sources of folic acid and iron in the diet, although this is difficult when protein sources are restricted. Inadequate intake and iron losses that may occur are managed by supplemental iron, usually ferrous sulfate. Providing adequate sources of ascorbic acid at the same time that iron-rich foods or supplements are given enhances the absorption. The medication recombinant human erythropoietin (r-HuEPO) corrects anemia (improving energy level and general well-being) and eliminates the need for frequent blood transfusions in patients with CKD. To support the formation of new red blood cells before r-HuEPO therapy, iron stores must be adequate. Iron supplements are required in conjunction with r-HuEPO.
ChatGPT said:
ChatGPT
Comparison of Acute Renal Failure (ARF) and Chronic Renal Failure (CRF) in Children
Acute Renal Failure (ARF) and Chronic Renal Failure (CRF) are distinct conditions with different pathophysiological mechanisms, clinical manifestations, and nursing management strategies. Here's a detailed comparison and contrast between the two.
Pathophysiology
Acute Renal Failure (ARF):
Onset: Rapid, usually occurring over hours to days.
Mechanism: Often caused by factors such as acute tubular necrosis (due to ischemia or toxins), prerenal causes (e.g., dehydration), or postrenal obstruction (e.g., urinary tract blockage).
Nephron Function: In ARF, there is a sudden decrease in kidney function leading to the accumulation of waste products. Nephrons may still be capable of recovery if the underlying cause is identified and treated early.
Retention of Waste Products: Increased blood urea nitrogen (BUN) and creatinine levels reflect impaired excretion.
Chronic Renal Failure (CRF):
Onset: Gradual, often developing over months to years.
Mechanism: Caused by ongoing renal injury (e.g., congenital malformations, glomerulonephritis, systemic diseases) leading to progressive nephron loss.
Nephron Function: More than 50% of nephron function is lost before clinical symptoms appear. The remaining nephrons compensate initially but ultimately fail.
Retention of Waste Products: Leads to uremia, characterized by increased BUN and creatinine, as well as electrolyte imbalances, including acidosis and disturbances in calcium and phosphate metabolism.
Clinical Manifestations
Acute Renal Failure (ARF):
Symptoms: Sudden onset of oliguria or anuria, edema, hypertension, nausea, vomiting, and altered mental status.
Electrolyte Imbalances: May show hyperkalemia, hyponatremia, and metabolic acidosis.
Overall Health: Symptoms are often more acute and severe due to rapid accumulation of toxins.
Chronic Renal Failure (CRF):
Symptoms: Gradual development of fatigue, pallor, growth retardation, poor appetite, bone pain, and neurological manifestations (e.g., confusion, seizures).
Electrolyte Imbalances: Characterized by hyperphosphatemia, hypocalcemia, and metabolic acidosis, leading to complications such as renal osteodystrophy and anemia.
Overall Health: Symptoms can be subtle and may not be noticeable until significant renal damage has occurred, with systemic effects developing gradually.
Nursing Management
Acute Renal Failure (ARF):
Monitoring: Close monitoring of vital signs, fluid intake and output, and laboratory values (BUN, creatinine, electrolytes).
Intervention: Immediate identification and treatment of underlying causes (e.g., fluid resuscitation, removal of nephrotoxins, management of obstruction).
Supportive Care: May require temporary dialysis to manage severe electrolyte imbalances or fluid overload.
Education: Educate families about the signs of renal failure and the importance of early intervention.
Chronic Renal Failure (CRF):
Monitoring: Regular monitoring of growth parameters, blood pressure, laboratory values (including hemoglobin, calcium, phosphorus, and vitamin D levels).
Intervention: Dietary management to limit protein, phosphorus, and sodium intake; supplementation of vitamins and minerals as needed; and medications to manage anemia (e.g., erythropoietin) and bone metabolism (e.g., vitamin D, phosphate binders).
Long-term Management: Prepare for eventual dialysis or kidney transplantation as renal function declines.
Education: Teach families about the importance of adhering to dietary restrictions, recognizing signs of complications, and understanding the disease process.
Summary
In summary, ARF is characterized by a rapid onset of kidney dysfunction with the potential for recovery, while CRF involves a gradual decline in kidney function with progressive and irreversible damage. The nursing management of ARF focuses on acute interventions and stabilization, whereas CRF requires long-term care strategies aimed at managing chronic complications and preserving quality of life. Both conditions require vigilant monitoring and education for families to support optimal health outcomes.
4o mini
You said:
Chronic Kidney Disease The kidneys are able to maintain the chemical composition of fluids within normal limits until more than 50% of functional renal capacity is destroyed by disease or injury. Chronic kidney disease (CKD) occurs when the diseased kidneys can no longer maintain the normal chemical structure of body fluids under normal conditions. Progressive deterioration over months or years produces a variety of clinical and biochemical disturbances that conclude in the clinical syndrome known as uremia. The final stage of CKD, ESRD, is irreversible. Treatment with dialysis or transplantation is required when the glomerular filtration rate decreases below 10% to 15% of normal. The pattern of renal dysfunction is remarkably uniform no matter what disease process initiates the advanced disease. Etiology A variety of diseases and disorders can result in CKD. The most common causes of CKD before age 5 years are congenital renal and urinary tract malformations (particularly renal hypoplasia and dysplasia and obstructive uropathy) and VUR. Glomerular and hereditary renal diseases predominate in children 5 to 15 years of age. The glomerular diseases that most commonly lead to CKD are chronic pyelonephritis, CGN, and glomerulonephropathy associated with systemic diseases such as anaphylactoid purpura and lupus erythematosus. Hereditary nephritis, congenital nephrotic syndrome, Alport syndrome, polycystic kidney, and several other hereditary disorders result in renal failure in childhood. Renal vascular disorders such as HUS, vascular thrombosis, or cortical necrosis are less common causes. Pathophysiology Early in the course of progressive nephron destruction, the child remains asymptomatic with only minimum biochemical abnormalities. Unless its presence is detected in the process of routine assessment, signs and symptoms that indicate advanced renal damage often emerge only late in the course of the disease. Midway in the disease process, as increasing numbers of nephrons are totally destroyed, and most others are damaged to varying degrees; the few that remain intact are hypertrophied but functional. These few normal nephrons are able to make sufficient adjustments to stresses to maintain reasonable degrees of fluid and electrolyte balance. Definitive biochemical examination at this time reveals restricted tolerance to excesses or restrictions. As the disease progresses to the end stage because of severe reduction in the number of functioning nephrons, the kidneys are no longer able to maintain fluid and electrolyte balance, and the features of uremia appear. The following sections briefly summarize the pathophysiology of specific biochemical abnormalities. Retention of Waste Products. Serum creatinine and BUN levels are utilized to evaluate renal function. Creatinine is a waste product of muscle catabolism. Because muscle mass is relatively stable, creatinine production is also stable. Most creatinine is filtered out by the kidneys and expelled in the urine. BUN (a by-product of protein breakdown) levels also increase as kidney function declines. BUN is a less precise marker than creatinine as is not produced at a stable rate and can be influenced by protein intake, hydration status and other factors (Lopez-Giacoman & Madero, 2015). Water and Sodium Retention. The damaged kidneys are able to maintain sodium and water balance under normal circumstances, although the few remaining functional nephrons are required to increase their rate of filtration and reabsorption in proportion to their numbers. The limitations of this capacity become apparent under stress. The nature of abnormalities in adjustment depends on the underlying renal disease. Infants and small children with kidney dysplasia or urinary obstructive disease tend to excrete large volumes of dilute urine low in sodium content. Children with glomerular disease tend to retain both sodium and water as a result of a greater reduction of glomerular filtration than of tubular reabsorption. Children with defective sodium reabsorption from tubular disease tend to lose sodium, with a corresponding osmotic water loss. Consequently, sodium excesses may cause edema and hypertension, whereas sodium deprivation can result in hypovolemia and circulatory failure. Only in ESRD is markedly reduced glomerular filtration inadequate to handle normal amounts of sodium and water. Retention of these substances leads to edema and vascular congestion. Hyperkalemia. Dangerous hyperkalemia is uncommon in CKD until the end stage. However, the kidneys are unable to adjust readily to increased ingestion of potassium, and they require a longer period to rid the body of this excess. Acidosis. A sustained metabolic acidosis is characteristic of CKD; it results from the damaged kidney's inability to excrete a normal load of metabolic acids generated by normal metabolic processes. There is reduced capacity of the distal tubules to produce ammonia and impaired reabsorption of bicarbonate. Despite continuous hydrogen ion retention and bicarbonate loss, the plasma pH is maintained at a level compatible with life by other buffering mechanisms, particularly the bone salt (see the following sections). Calcium and Phosphorus Disturbances. Calcium and phosphorus homeostasis are affected by CKD. Profound and complex disturbances in the metabolism of these substances result in significant bone demineralization and impaired growth. This appears to be related to several factors (Box 24.8). These complex disturbances in calcium, phosphorus, and bone metabolism produce growth arrest or delay; bone pain; and deformities known as renal osteodystrophy, sometimes called renal rickets, because the disorganization of bone growth and demineralization are similar to that caused by vitamin D–resistant rickets. Box 24.8 Factors Related to Bone Demineralization in Chronic Renal Failure • In a state of acidosis there is dissolution of the alkaline salts of bone, which serve as buffers, and the release of phosphorus and calcium into the bloodstream. • Reduced glomerular filtration and excretion of inorganic phosphate lead to an elevation of plasma phosphate with a concomitant decrease in serum calcium. • Decreased serum calcium concentration stimulates the secretion of parathyroid hormone, which results in reabsorption of calcium from bones. Under normal circumstances parathyroid hormone inhibits the tubular reabsorption of phosphates. • Diseased kidneys are unable to complete the synthesis of vitamin D to its most active form, 1,25-dihydroxycholecalciferol, which is necessary for the absorption of calcium from the gastrointestinal tract and deposition of calcium in bone. This acquired resistance to vitamin D decreases calcium absorption, permits retention of phosphorus, and contributes to secondary hyperparathyroidism. Anemia. A consistent feature of CKD is anemia, which appears to result from several factors (Box 24.9). Box 24.9 Causes of Anemia in Chronic Renal Failure • Shortened life span of red blood cells caused by some extracorpuscular factor associated with the uremic state • Impaired red blood cell production resulting from decreased production of erythropoietin • Blood loss related to increased tendency to bleed, associated with a prolonged bleeding time, probably related to impaired platelet function and laboratory blood samples • Hyperparathyroidism • Hypersplenism, which may be related to silicone deposition (from dialysis blood lines) and granuloma formation in the spleen • Diseases related to hemolytic anemia, such as systemic lupus erythematosus and sickle cell disease Growth Disturbance. One of the most striking effects of CKD in childhood, and one that can have profound psychologic and social consequences for the developing child, is delayed growth. The cause is poorly understood but may be related to nutritional and biochemical factors (Box 24.10). Box 24.10 Probable Causes of Growth Failure in Chronic Renal Failure • Renal osteodystrophy • Poor nutrition associated with dietary restrictions (especially protein) and loss of appetite • Biochemical abnormalities associated with renal failure, such as sustained acidosis or renal sodium wasting • Hypertension • Corticosteroid treatment • Tissue resistance to growth hormone • Trace mineral and vitamin deficiencies Sexual maturation may be delayed or may not occur in children with CKD, and secondary amenorrhea commonly develops in girls past puberty. CKD can also cause sexual dysfunction by creating imbalances in gonadal hormone levels. Decreased testosterone levels impair spermatogenesis in males; decreased estrogen, luteinizing hormone, and progesterone cause anovulation and menstrual irregularities (usually amenorrhea) in females. Autonomic neuropathy and anemia are also factors that can alter sexual function. Other Disturbances. Children with CKD are more susceptible to infection, especially pneumonia, UTI, and septicemia, although the reason for this is not entirely clear. Hyperventilation, a manifestation of the respiratory compensatory mechanism for metabolic acidosis, and pulmonary edema may contribute to upper respiratory tract infection. These children become extraordinarily sensitive to changes in vascular volume that may cause, in addition to pulmonary overload, cerebral symptoms and circulatory manifestations such as hypertension and cardiac failure. Numerous neurologic manifestations appear with advanced renal failure, although no specific toxin or biochemical defect has been identified. However, disturbances in enzyme function, disturbances in water and electrolyte balance, altered calcium ion concentration, hypertension, and accumulation of various “uremic toxins” have been implicated. Clinical Manifestations The first symptom of CKD may be loss of normal energy and increased fatigue on exertion. For example, the child may prefer quiet, passive activities rather than participation in more active games and outdoor play. The child is usually somewhat pale, but the change is often so subtle that it may not be evident to parents or others. Blood pressure is sometimes elevated. Growth is affected early in the development of CKD, and falling behind on the growth chart is often the first measurable sign. Other manifestations may appear as the disease progresses. The child does not eat as well (especially breakfast), shows less interest in normal activities such as schoolwork or play, and has a decreased or increased urinary output and a compensatory intake of fluid. For example, a child who has achieved bladder control may wet the bed at night. Pallor becomes more evident as the skin develops a characteristic sallow, muddy appearance as a result of anemia and deposition of urochrome pigment in the skin. The child may complain of headache, muscle cramps, and nausea. Other signs and symptoms include weight loss, facial puffiness, malaise, bone or joint pain, growth retardation, dryness or itching of the skin, bruised skin, and sometimes sensory or motor loss. Amenorrhea is common in adolescent girls. Therapy is generally initiated before the appearance of the uremic symptoms, although on some occasions the symptoms may be observed. Manifestations of untreated uremia reflect the progressive nature of the homeostatic disturbances and general toxicity. Gastrointestinal symptoms include loss of appetite, nausea, and vomiting. Bleeding tendencies are apparent in bruises, bloody diarrheal stools, stomatitis, and bleeding from the lips and mouth. Intractable itching occurs, probably related to a number of factors, including dry skin and hyperparathyroidism (Wojtowicz-Prus, Kiliś-Pstrusińska, Reich, et al., 2016). Deposits of urea crystals may appear on the skin as uremic frost but are seldom seen because of the availability of dialysis and transplantation. There may be an unpleasant uremic odor to the breath. Respirations become deeper as a result of metabolic acidosis, and circulatory overload is manifested by hypertension, congestive heart failure, and pulmonary edema. Progressive confusion, dulling of the sensorium, and ultimately coma are signs of neurologic involvement. Other signs may include tremors, muscular twitching, and seizures. Diagnostic Evaluation The diagnosis of CKD is usually suspected on the basis of any of a number of clinical manifestations, a history of prior renal disease, or biochemical findings. The onset is usually gradual, and the initial signs and symptoms are vague and nonspecific. Laboratory and other diagnostic tools and tests are of value in assessing the extent of renal damage, biochemical disturbances, and related physical dysfunction. Often, they can help establish the nature of the underlying disease and differentiate between other disease processes and the pathologic consequences of renal dysfunction. Therapeutic Management Classification of CKD as stage 1 (GFR ≥90) through stage 5 (GFR <15 or dialysis) helps with evaluation and management decisions through the use of medical protocols such as the National Kidney Foundation Kidney Disease Outcomes Quality Initiative evidence-based clinical practice guidelines (http://www.kidney.org/professionals/KDOQI). The goals of management are to maximize effective renal function, maintain body fluid and electrolyte balance within acceptable limits, treat systemic complications, and promote as active and normal a life as possible for the child for as long as possible. This becomes increasingly difficult as the disease progresses toward end stage. Therapeutic measures designed to relieve one manifestation may negatively affect another. For example, antihypertensive agents may further impair renal function. Activity. Allow children unrestricted activity and to set their own limits regarding rest and extent of exertion. Encourage them to attend school. If the effort is too great, home tutoring can be arranged. Diet. Regulation of diet has been seen as the most effective means, short of dialysis, for reducing the quantity of materials that require renal excretion. The goal of the diet in renal failure is to provide sufficient calories and protein for growth while minimizing the excretory demands made on the kidney, to limit metabolic bone disease (osteodystrophy), and to minimize fluid and electrolyte disturbances. Dietary protein intake is limited to the recommended dietary allowance (RDA) for the child's age. Restriction of protein intake below the RDA is believed to negatively affect growth and neurodevelopment. Dietary phosphorus may need to be restricted. Remember that any attempt to restrict dietary intake in children potentially restricts caloric intake and can limit growth. Protein in the diet should include foods of high biologic value. When given with meals, substances that bind phosphorus in the intestines prevent its absorption and allow a more liberal intake of phosphorus-containing protein. Sodium and water are not usually limited unless there is evidence of edema or hypertension. Potassium is not restricted as long as creatinine clearance remains at acceptable limits (30 to 35 ml/min). However, restrictions are instituted for patients with oliguria or anuria. Restrictions of any or all of these minerals may be imposed in later stages or at any time in which factors cause abnormal serum concentrations. Because of modified dietary intake, altered metabolism, and poor appetite, some dietary supplementation is usually needed. Because fat-soluble vitamins can accumulate in patients with CKD, vitamins A, E, and K are not supplemented beyond normal dietary intake. Active and/or inactive forms of vitamin D are prescribed, and water-soluble vitamin supplementation may be required if the diet is inadequate. Other dietary needs are discussed in relation to osteodystrophy and anemia. Dietary management of the child with renal failure is a difficult and complex problem that necessitates collaboration with a registered dietitian who is knowledgeable about pediatric nutrition and the impact of renal failure. Osteodystrophy. Measures directed at prevention or correction of the calcium/phosphorus imbalance are reduction of dietary phosphorus, administration of a phosphorus-binding agent, provision of supplemental calcium, control of acidosis, and administration of an active and/or inactive form of vitamin D. The reduction of protein and milk intake can control dietary phosphorus. Oral administration of phosphorus-binding agents, which combine with the phosphorus to decrease gastrointestinal absorption and thus the serum levels of phosphate, can further reduce phosphorus levels. Calcium carbonate preparations can be used as phosphorus binders. These medications act as phosphate binders, calcium supplements, and alkalizing agents. Calcium carbonate preparations can be given with meals to bind phosphorus if the child is hyperphosphatemic or mildly hypocalcemic. If given 1 to 2 hours after meals, they act as calcium supplements for children with stable phosphorus but low calcium levels. Calcium acetate can also be used. Newer, iron-based binders can be used when there is concern for hypercalcemia (Stormont, McCoy, Bashir, et al., 2016). Aluminum hydroxide gels are effective phosphorus binders but have been shown to cause aluminum loading when used in children with renal failure. Aluminum intoxication leads to altered sensorium, an inability to talk, ataxia, seizures, and severe bone disease. Secondary hyperparathyroidism (evidenced by an elevated intact PTH level), in a child with normal phosphate, calcium and 25OH vitamin D levels requires treatment with an active form of vitamin D. Oral medications such as calcitriol (Rocaltrol) increase absorption of calcium through the gastrointestinal tract. The serum calcium level is monitored frequently during periods when the drugs are being initiated or changed to detect hypercalcemia. Intact parathyroid hormone levels are measured every 2 to 3 months with target levels based on the stage of CKD. Osseous deformities that result from renal osteodystrophy, especially those related to ambulation, are troublesome and require correction if they occur. Careful attention to the management of osteodystrophy and bone growth can prevent deformities in some children. Acidosis. Pharmacologic treatment of acidosis is initiated early in children who have chronic renal insufficiency. In addition to reducing the formation of metabolic acids by avoiding excessive dietary protein intake, alkalizing agents such as sodium bicarbonate or a combination of sodium and potassium citrate (Bicitra, Polycitra, or Shohl solution) alleviate acidosis. Correction of acidosis is best attempted after calcium levels are elevated because rapid correction may precipitate tetany in a hypocalcemic child. Anemia. Because the anemia associated with renal failure is related to decreased production of erythropoietin, it usually cannot be successfully managed with hematinic agents. Provide sufficient sources of folic acid and iron in the diet, although this is difficult when protein sources are restricted. Inadequate intake and iron losses that may occur are managed by supplemental iron, usually ferrous sulfate. Providing adequate sources of ascorbic acid at the same time that iron-rich foods or supplements are given enhances the absorption. The medication recombinant human erythropoietin (r-HuEPO) corrects anemia (improving energy level and general well-being) and eliminates the need for frequent blood transfusions in patients with CKD. To support the formation of new red blood cells before r-HuEPO therapy, iron stores must be adequate. Iron supplements are required in conjunction with r-HuEPO. Hypertension. Hypertension of advanced renal disease may be managed initially by cautious use of a low-sodium diet, fluid restriction, and perhaps diuretics such as thiazides or furosemide. Strict restriction of sodium intake may be necessary in patients with oliguria. Severe hypertension may require the combination of a beta blocker and a vasodilator (propranolol and hydralazine). Other drugs that may be used include nifedipine, atenolol, minoxidil, prazosin, captopril, or labetalol, either singly or in combinations. Growth Retardation. One major consequence of CKD is growth retardation. Children with onset of renal failure earlier in life have more severe growth impairment than those diagnosed later (Rodig, McDermott, Schneider, et al., 2014). These children grow poorly both before and after initiation of dialysis. Assurance of adequate nutritional intake, correction of fluid and electrolyte imbalances, anemia, and metabolic acidosis should be undertaken. The use of recombinant human growth hormone has shown marked acceleration in growth velocity in children with growth retardation secondary to CKD (Rees, 2016). Miscellaneous Complications. Intercurrent infections are treated with appropriate antimicrobials. Most of these drugs are excreted through the kidneys; therefore the dosage is usually reduced in proportion to the decrease in renal function, and the interval between doses is extended in these children to avoid possible toxic effects from accumulation. Any drug eliminated through the kidneys is administered with caution. Serum levels of ototoxic or nephrotoxic drugs (e.g., aminoglycosides or vancomycin) are assessed regularly to ensure a safe, nontoxic level. Dental defects are common in children with chronic kidney disease; the earlier the onset of the disease, the more severe the dental manifestations. These defects include hypoplasia, hypomineralization, tooth discoloration, alteration in the size and shape of teeth, malocclusion (secondary to deficient skeletal growth), ulcerative stomatitis, occasional oral hematomas, and an increase in calcific deposits around the teeth. Regular dental care is especially important in these children. Other nondental complications are treated symptomatically—for example, chlorpromazine (Thorazine) or prochlorperazine (Compazine) is given for nausea, antiepileptics are given for seizures, and diphenhydramine (Benadryl) is given for pruritus. Once a child reaches CKD stage 5, death can occur unless waste products and toxins are removed from body fluids by dialysis or kidney transplantation. Since the adaptation of these techniques for infants and small children, the outlook for these patients has improved remarkably. In cases in which the patient has other serious illnesses or organ system failures and aggressive care is considered futile, the appropriate end-of-life recommendation may be for palliative care and comfort measures only. (See Quality Patient Outcomes box.) Quality Patient Outcomes Chronic Renal Failure • Sufficient calories and protein for growth maintained • Excretory demands made on the kidney are limited • Metabolic bone disease (osteodystrophy) minimal • Fluid and electrolyte disturbances managed • Hypertension managed • Growth retardation treated Nursing Care Management
ChatGPT said:
ChatGPT
Chronic Kidney Disease (CKD) is a condition characterized by the progressive loss of kidney function over time, often leading to end-stage renal disease (ESRD), where renal replacement therapy, such as dialysis or transplantation, becomes necessary. The following details outline various aspects of CKD, including etiology, pathophysiology, clinical manifestations, diagnostic evaluation, and therapeutic management.
Etiology
The causes of CKD vary by age. In children under 5 years, the most common causes include:
Congenital renal and urinary tract malformations (e.g., renal hypoplasia, dysplasia, obstructive uropathy).
Vesicoureteral reflux (VUR).
For children aged 5 to 15 years, glomerular and hereditary renal diseases become more prevalent, including:
Chronic pyelonephritis.
Chronic glomerulonephritis (CGN).
Hereditary conditions such as Alport syndrome and congenital nephrotic syndrome.
Renal vascular disorders, although they are less common.
Pathophysiology
In the early stages of CKD, children often remain asymptomatic despite some biochemical changes. As nephron damage progresses:
The surviving nephrons undergo hypertrophy to maintain fluid and electrolyte balance.
As nephrons continue to be destroyed, uremic symptoms develop, marked by the retention of waste products, water, sodium, and imbalances in electrolytes and acids.
Key biochemical disturbances include:
Waste Product Retention: Elevated serum creatinine and BUN indicate declining kidney function.
Water and Sodium Retention: Resulting in edema and hypertension, especially in ESRD.
Hyperkalemia: Typically occurs in later stages due to the kidneys' inability to excrete potassium.
Metabolic Acidosis: A hallmark of CKD, due to impaired excretion of hydrogen ions and bicarbonate reabsorption.
Calcium and Phosphorus Disturbances: Can lead to renal osteodystrophy due to complex alterations in mineral metabolism.
Clinical Manifestations
Early signs of CKD may include:
Fatigue and decreased energy.
Pallor due to anemia.
Hypertension.
Growth impairment, observable on growth charts.
As the disease progresses, additional symptoms can manifest, including:
Decreased appetite and altered activity levels.
Urinary output changes (e.g., enuresis).
Symptoms of uremia, such as nausea, vomiting, and itching.
Neurologic symptoms like confusion or seizures may occur in advanced stages.
Diagnostic Evaluation
Diagnosis typically involves:
Clinical Assessment: Identification of symptoms and history of renal disease.
Laboratory Tests: Assessment of renal function through serum creatinine and BUN levels, urine analysis, and imaging studies as needed.
GFR Classification: Determining the stage of CKD based on the glomerular filtration rate (GFR).
Therapeutic Management
Management goals include maximizing renal function, maintaining fluid and electrolyte balance, treating systemic complications, and promoting normal development and quality of life. Key components include:
Activity
Encourage unrestricted activity tailored to the child's stamina.
Facilitate school attendance and home tutoring if necessary.
Diet
Tailored to provide adequate calories and protein while minimizing renal excretory demands.
May involve restrictions on protein, phosphorus, and sodium based on disease stage and symptoms.
Monitoring and supplementation of vitamins and minerals (excluding excess fat-soluble vitamins).
Osteodystrophy Management
Address calcium and phosphorus imbalances through diet, phosphorus-binding agents, and vitamin D supplementation.
Acidosis Treatment
Alkalizing agents like sodium bicarbonate to correct metabolic acidosis.
Anemia Management
Erythropoietin therapy to stimulate red blood cell production, coupled with iron supplementation.
Hypertension Management
Lifestyle modifications with dietary sodium restriction, fluid management, and pharmacological treatment as needed.
Growth and Development Support
Focus on nutrition, correction of imbalances, and potentially growth hormone therapy.
Monitoring for Complications
Regular assessments for intercurrent infections and other complications, adjusting medication dosages based on renal function.
Nursing Care Management for Children with Chronic Kidney Disease (CKD)
Overview: Children with CKD rely heavily on medications and artificial interventions to maintain their health. The progressive nature of the disease and its management can impose significant stress on both the child and their family. Nursing care plays a vital role in supporting these families through various stages of the disease, from renal insufficiency to uremia, dialysis, or transplantation.
Multidisciplinary Approach: Effective care requires a coordinated effort from a multidisciplinary team, which may include:
Medical Professionals: Nephrologists, pediatricians
Nursing Staff: Nurses specializing in pediatric nephrology
Social Workers: To address emotional and financial concerns
Child Life Specialists: To provide support through play and education
Physical and Occupational Therapists: To help with physical development and daily activities
Dietitians: To manage dietary restrictions and nutritional needs
Psychologists/Psychiatrists: To support mental health
Family-Centered Care: The emotional and psychological impact of CKD on families can be profound. Nurses must recognize that while the ill child requires attention, family dynamics and priorities must also be considered. Families often face competing needs, such as work and the needs of siblings, which may influence their ability to comply with treatment schedules.
Key Considerations in Nursing Care:
Understanding Family Dynamics:
Each family's ability to cope with CKD is influenced by factors such as cultural background, socioeconomic status, and interpersonal relationships.
Nurses should facilitate open communication with families, helping them prioritize needs and navigate the complexities of care.
Support Through Treatment:
Frequent hospital visits and medical procedures can be overwhelming. Nurses should prepare families for what to expect and help them understand the treatment process.
Help families manage medication regimens, which can be complex and challenging to maintain over time.
Dietary Education:
Nurses should assist families in understanding dietary restrictions and modifications required for the child’s condition, including sodium and potassium limitations.
Educational resources should be provided to help parents read food labels and select appropriate meals.
Addressing Psychological Needs:
Encourage families to express their feelings about the diagnosis and treatment, and be alert for signs of anxiety and depression.
Offer support strategies and coping mechanisms to both children and parents, especially as they navigate the emotional challenges of chronic illness.
School Support:
Advocate for the child’s educational needs, ensuring they receive appropriate accommodations in school.
Collaborate with teachers and school staff to create a supportive learning environment, addressing attendance issues due to illness.
Financial Resources:
Educate families about available financial resources, including insurance coverage and community assistance programs.
Familiarize yourself with organizations like the National Kidney Foundation that provide educational materials and support for families.
Emotional Impact and Coping Strategies:
Children with CKD may experience noticeable physical changes that affect their self-image and interactions with peers. Common challenges include:
Body Image Issues: Encourage children to develop positive coping strategies and resilience in dealing with teasing or social stigma.
Behavioral Changes: Some children may exhibit regression in behavior due to stress and illness. Address these changes with supportive interventions.
19. Discuss laboratory findings associated with acute renal failure
Laboratory Findings Associated With Acute Renal Failure
Clinical ProblemMechanismClinical Considerations | ||
Azotemia | Ongoing protein catabolism | Lower rate of production in neonates and individuals with depleted protein stores. |
Elevated blood urea nitrogen levels | Significantly decreased excretion; increased in situations involving large amounts of necrotic tissue or extravasated blood. | |
Elevated plasma creatinine levels | Continued production; significantly decreased excretion; production less affected by other factors. More sensitive measure of intensity of azotemia; levels are low in neonates due to small muscle mass relative to size. | |
Metabolic acidosis | Continued endogenous acid production | Significantly decreased excretion; depletion of extracellular and intracellular fluid buffers; compensatory hyperventilation; opisthonos; major threat to life. |
Hyponatremia | Dilution of extracellular fluid | Decreased excretion of water; may develop cerebral signs. |
Hyperkalemia | Ongoing protein catabolism | Decreased excretion compounded by metabolic acidosis; most important electrolyte to consider in acute renal failure; may contribute to cardiac arrhythmia; with electrocardiogram changes, poses a major threat to life. Loss may occur from the gastrointestinal tract. |
Hypocalcemia | Associated with metabolic acidosis and hyperphosphatemia | During alkali therapy, may cause tetany. |
20. Describe therapeutic management and nursing indications for care related to oliguria, fluid and calories, hyperkalemia, hypertension, and other complications such as anemia, seizures, coma, cardiac failure, and pulmonary edema associated with acute renal failure.
1. Oliguria Management:
Therapeutic Management:
Monitor urine output carefully; define oliguria as less than 0.5 mL/kg/hour.
Assess for signs of fluid overload (edema, hypertension).
Administer diuretics (e.g., furosemide) if prescribed, based on the patient's fluid status and renal function.
Consider dialysis if oliguria persists and leads to complications.
Nursing Indications:
Maintain strict intake and output records.
Weigh the child daily to monitor fluid status.
Observe for symptoms of dehydration or fluid overload.
2. Fluid Management:
Therapeutic Management:
Calculate fluid requirements based on the child’s weight, age, and clinical status.
Administer fluids cautiously to avoid overload; consider fluid restrictions if necessary.
Nursing Indications:
Monitor vital signs for signs of fluid overload (e.g., increased blood pressure, tachycardia).
Assess lung sounds and respiratory status to detect pulmonary edema.
Provide education to families about fluid restrictions and signs of fluid imbalance.
3. Caloric Management:
Therapeutic Management:
Provide a diet high in carbohydrates and fats, with restricted protein intake as prescribed.
Monitor nutritional status and weight to ensure adequate caloric intake.
Nursing Indications:
Collaborate with a dietitian to develop meal plans.
Educate families on appropriate food choices and reading food labels.
Monitor for signs of malnutrition or growth failure.
4. Hyperkalemia Management:
Therapeutic Management:
Monitor serum potassium levels regularly; normal range is 3.5–5.0 mEq/L.
Administer medications such as calcium gluconate, insulin with glucose, or sodium bicarbonate to stabilize cardiac membranes and lower potassium levels.
Consider dialysis if potassium levels are dangerously high or if other treatments are ineffective.
Nursing Indications:
Assess the child for signs of hyperkalemia (e.g., muscle weakness, ECG changes).
Educate families about potassium-restricted diets and foods to avoid (e.g., bananas, oranges, potatoes).
5. Hypertension Management:
Therapeutic Management:
Monitor blood pressure regularly; normal range varies by age.
Administer antihypertensive medications as prescribed (e.g., ACE inhibitors, calcium channel blockers).
Nursing Indications:
Educate families on the importance of regular blood pressure monitoring.
Encourage lifestyle modifications such as dietary changes (low sodium).
6. Management of Other Complications:
Anemia:
Therapeutic Management: Administer erythropoiesis-stimulating agents (e.g., epoetin alfa) and iron supplements as needed.
Nursing Indications: Monitor hemoglobin and hematocrit levels; educate families on signs of anemia.
Seizures and Coma:
Therapeutic Management: Ensure seizure precautions; administer anticonvulsants as prescribed.
Nursing Indications: Monitor neurological status; assess for any triggers or changes in consciousness.
Cardiac Failure:
Therapeutic Management: Monitor for signs of heart failure; administer diuretics and medications to manage heart function.
Nursing Indications: Monitor vital signs and fluid status closely.
Pulmonary Edema:
Therapeutic Management: Administer oxygen as needed; consider diuretics to manage fluid overload.
Nursing Indications: Monitor respiratory status and lung sounds for signs of fluid accumulation.
21. Describe the etiology of CRF.
Causes of CKD by Age Group
Before Age 5 Years:
Congenital Renal and Urinary Tract Malformations:
Renal Hypoplasia and Dysplasia: These are conditions where the kidneys are underdeveloped or malformed, leading to impaired kidney function.
Obstructive Uropathy: This occurs when there is a blockage in the urinary tract that impedes the flow of urine, potentially causing kidney damage.
Vesicoureteral Reflux (VUR): A condition where urine flows backward from the bladder into the ureters and sometimes into the kidneys, which can lead to recurrent urinary tract infections and kidney damage.
Ages 5 to 15 Years:
Glomerular Diseases:
Chronic Pyelonephritis: A recurring kidney infection that can lead to scarring and loss of kidney function.
Chronic Glomerulonephritis (CGN): A progressive kidney disease that affects the glomeruli (filtering units of the kidney), resulting in inflammation and scarring.
Glomerulonephropathy Associated with Systemic Diseases:
Anaphylactoid Purpura (Henoch-Schönlein Purpura): A small-vessel vasculitis that can affect the kidneys.
Systemic Lupus Erythematosus (SLE): An autoimmune disease that can lead to kidney inflammation and damage.
Hereditary Renal Diseases:
Hereditary Nephritis: A genetic disorder affecting the glomeruli, often leading to progressive kidney failure.
Congenital Nephrotic Syndrome: A genetic condition causing excessive protein loss in urine and resulting in kidney failure.
Alport Syndrome: A genetic condition characterized by kidney disease, hearing loss, and eye abnormalities.
Polycystic Kidney Disease (PKD): A hereditary disorder causing numerous cysts to grow in the kidneys, leading to kidney enlargement and dysfunction.
Less Common Causes:
Renal Vascular Disorders:
Hemolytic Uremic Syndrome (HUS): A condition often triggered by infections that can lead to kidney failure.
Vascular Thrombosis: The formation of a blood clot in a renal vein can disrupt kidney function.
Cortical Necrosis: A rare condition characterized by the death of the kidney cortex, often due to severe hypotension or other critical illnesses.
22. Discuss indications for hospice care, dialysis, and renal transplantation.
Indications for Hospice Care, Dialysis, and Renal Transplantation
Managing chronic kidney disease (CKD) and its complications in children requires careful consideration of various treatment modalities, including hospice care, dialysis, and renal transplantation. The choice of treatment often depends on the child's condition, the nature of the renal failure, and the family's preferences and circumstances.
1. Indications for Hospice Care
Hospice care is a compassionate approach for children with terminal illnesses, including those with advanced CKD, who are not responding to curative treatments. Key indications for hospice care include:
End-Stage Renal Disease (ESRD): When the child is in the final stages of CKD and dialysis is not an option or is no longer effective.
Palliative Needs: Focus on relieving symptoms and improving the quality of life rather than prolonging life. This includes pain management and psychological support.
Family Considerations: Families may choose hospice care to provide a supportive environment that addresses emotional and spiritual needs, allowing them to spend quality time together in the child’s final days.
Non-Compliance with Treatment: Children who refuse dialysis or have severe comorbidities that complicate treatment may benefit from hospice care.
Limited Life Expectancy: When a child’s prognosis is poor, with a life expectancy of typically six months or less, hospice care can provide supportive measures and comfort.
2. Indications for Dialysis
Dialysis is indicated for children with acute or chronic renal failure when conservative management is insufficient. The decision to initiate dialysis is based on several criteria:
Acute Conditions: Dialysis may be required in acute renal failure due to:
Severe metabolic acidosis.
Severe electrolyte imbalances (e.g., hyperkalemia, severe hypernatremia).
Severe volume overload (e.g., congestive heart failure with fluid overload).
Acute poisoning or toxic ingestions.
Tumor lysis syndrome in oncology patients.
Chronic Conditions: For children with chronic kidney disease, indications for dialysis include:
Biochemical abnormalities: Elevated blood urea nitrogen (BUN), hyperphosphatemia, and high potassium levels that are life-threatening.
Clinical manifestations: Deterioration of central nervous system function, congestive heart failure unresponsive to medical management, growth failure, severe osteodystrophy, and insufficient caloric intake.
Inability to perform daily activities: Indicating a significant impact on quality of life.
Dialysis is typically initiated when the child is in end-stage renal failure (ESRF) unless urgent intervention is needed in acute settings.
3. Indications for Renal Transplantation
Renal transplantation is the preferred treatment for children with end-stage renal disease when feasible, offering the best potential for long-term survival and quality of life. Indications for renal transplantation include:
End-Stage Renal Disease: Children with irreversible kidney failure who are not likely to benefit from dialysis or have a poor prognosis with dialysis alone.
Age and Growth Considerations: Transplantation is often more beneficial for younger children, as it allows for improved growth and development compared to remaining on dialysis.
Good Overall Health: Candidates must be in good general health to withstand the surgery and the immunosuppressive therapy that follows transplantation.
Absence of Contraindications: Certain medical conditions or factors may disqualify a child from being a transplant candidate, including active infections, malignancies, or severe comorbidities.
Family Support and Compliance: A strong support system and the ability to adhere to the postoperative regimen are critical for the success of a transplant.
23. Identify the indications and nursing care management for renal replacement therapy via hemodialysis, peritoneal dialysis, and continuous venovenous hemofiltration.
Hemodialysis:
Indications: Preferred for acute conditions like life-threatening hyperkalemia or poisoning. Suitable for children living close to a dialysis center, especially those who are noncompliant with PD.
Peritoneal Dialysis
Indications: Effective for acute conditions and chronic management in children, particularly those who prefer fewer dietary restrictions and gentler processes. It is often performed at home.
Continuous Venovenous Hemofiltration (CVVH)
Indications:
Acute Kidney Injury (AKI): Especially in critically ill patients who require continuous renal replacement therapy.
Fluid Overload: Patients who cannot tolerate rapid fluid removal via traditional hemodialysis.
Severe Metabolic Disturbances: Conditions like hyperkalemia and metabolic acidosis needing gradual correction.
Pediatric Patients: Particularly those with fluid overload from surgical procedures without severe biochemical abnormalities.
24. Discuss renal transplantation issues, including selection of donor tissue, immunosuppression, and signs of rejection.
Kidney Transplantation: Overview and Nursing Management
Kidney transplantation is the preferred form of renal replacement therapy for children with end-stage renal disease (ESRD). It offers a better quality of life compared to dialysis, allowing children to return to a more normal lifestyle.
Sources of Donor Kidneys
Living Donor: Typically a family member (parent, grandparent, sibling).
Deceased Donor: Kidneys are procured from individuals who have consented to organ donation after brain death.
Recipient Selection Criteria
Criteria for selecting transplant candidates vary by center, with no strict uniformity. Factors affecting candidacy may include:
Mental Health: Emotional stability and ability to adhere to medication regimens are critical.
Medical Conditions: Recipients must be cancer-free for a specified period if they previously had malignancies.
Infections: Any generalized infections must be treated prior to transplantation.
Bladder Capacity: Adequate bladder function is essential for transplant success.
Transplant Procedure
The transplanted kidney is usually placed in the extraperitoneal space (anterior iliac fossa).
Surgical connections:
Renal Artery: Anastomosed to the internal iliac or hypogastric artery.
Renal Vein: Anastomosed to the hypogastric vein.
Ureter: Implanted into the bladder or anastomosed to the recipient's ureter.
Immunological Considerations
Tissue Compatibility: The closer the genetic match, the better the chances for graft survival. Identical twins are the best match, followed by siblings, parents, and grandparents.
Immunosuppressant Therapy: Essential to prevent rejection. Common regimens include:
Prednisone: An anti-inflammatory that suppresses immune responses.
Tacrolimus: Reduces T-cell activation.
Mycophenolate: Inhibits lymphocyte proliferation.
Monitoring for Rejection:
Hyperacute Rejection: Occurs immediately post-transplant and is irreversible.
Acute Rejection: Can occur days to months post-transplant; may be treated with corticosteroids or monoclonal antibodies.
Chronic Rejection: Characterized by gradual decline in kidney function; often irreversible.
25. Identify common defects of the genitourinary tract in infants and young children.
1. Phimosis
Definition: A condition where the preputial opening of the foreskin cannot be retracted over the glans penis.
Normal Development: Typically normal in infants; often resolves naturally as the child grows.
Complications: Can lead to urinary obstruction or balanitis (inflammation/infection of the foreskin).
Treatment: Steroid cream application or circumcision for severe cases.
Nursing Care: Maintain proper hygiene, avoid forcible retraction to prevent scarring, and monitor for signs of paraphimosis (emergency situation).
2. Hydrocele
Definition: Accumulation of fluid in the scrotum, leading to painless swelling.
Types:
Communicating: Fluid communicates with the peritoneal cavity (often resolves spontaneously).
Noncommunicating: No connection with the peritoneum (may require treatment if symptomatic).
Management: Surgical intervention for persistent communicating hydroceles after age 1; usually outpatient procedures.
Nursing Care: Educate parents on post-operative care, swelling management, and activity restrictions.
3. Cryptorchidism
Definition: Failure of one or both testes to descend into the scrotum.
Prevalence: More common in premature infants; spontaneous descent often occurs in the first 6 months.
Complications: Increased risk of inguinal hernia, testicular torsion, infertility, and testicular cancer.
Diagnosis: Physical examination to differentiate between nonpalpable and retractile testes; laparoscopic surgery for further evaluation.
Therapeutic Management: Orchiopexy recommended if no descent occurs by 6 months; typically performed between 6-24 months.
Nursing Care: Postoperative care to prevent infection, manage pain, and educate on testicular self-examination.
4. Hypospadias
Definition: Congenital abnormality where the urethral opening is located on the ventral side of the penis.
Incidence: Affects approximately 1 in 300 males, with higher recurrence in first-degree relatives.
Severity: Based on the location of the urethral opening and associated penile curvature (chordee).
Associated Conditions: Up to 10% of infants with hypospadias may also have cryptorchidism.
Management: Surgical correction, typically between 6-18 months of age.
Nursing Care: Pre- and postoperative education, monitoring for infection, and ensuring proper wound care.
Epispadias and Exstrophy Complex (EEC)
Overview
Bladder exstrophy is a severe congenital defect affecting the musculoskeletal, urinary, reproductive, and sometimes gastrointestinal systems. It is part of the exstrophy-epispadias complex (EEC), which includes:
Epispadias: The least severe condition where the urethra fails to close properly, leading to an exposed dorsal urethra.
Bladder Exstrophy: A more severe condition characterized by an "inside-out" bladder, where the inner surface is exposed through an abdominal wall defect. Associated anomalies may include diastasis of the symphysis pubis, low-set umbilicus, anteriorly displaced anus, genital defects, and inguinal hernia.
Cloacal Exstrophy: The most severe form, involving both bladder and intestinal exstrophy, anal atresia, omphalocele, hypoplasia of the colon, and genital anomalies. It is associated with spinal dysraphism and renal abnormalities.
Prevalence
Cloacal Exstrophy: 1 in 131,579 live births, more common in females.
Bladder Exstrophy: 2.07 per 100,000 live births, nearly twice as common in males than females.
Pathophysiology
The etiology of bladder exstrophy is linked to a complex embryologic defect in abdominal wall development. This condition leads to various genital and urinary tract anomalies:
Males: Epispadias, upward curvature of the penis, cryptorchidism, and potential inguinal hernias.
Females: Epispadias, bifid clitoris, small labia minora, and a shortened vagina. Cloacal exstrophy may also result in a bifid or duplicated uterus and significant genital anomalies.
26. Describe disorders of sex development.
Disorders of Sex Development (DSD)
Definition and Presentation
Disorders of Sex Development (DSD) refer to a group of conditions in which there is a discrepancy between the chromosomal, gonadal, and anatomical sex of an individual. Infants with DSD may exhibit ambiguous genitalia at birth, making it difficult to assign a clear gender. Common presentations of DSD include:
Bilateral Cryptorchidism: Undescended testicles in a male infant.
Perineal Hypospadias with Bifid Scrotum: An abnormality where the urethra opens on the underside of the penis.
Clitoromegaly: Enlargement of the clitoris.
Posterior Labial Fusion: Fusion of the labia, often observed in females.
Phenotypic Female Appearance with Palpable Gonad: A female external appearance but with an undescended testis.
Hypospadias with Unilateral Nonpalpable Gonad: A urethral opening that is not at the tip of the penis alongside a non-visible testicle.
Additionally, chromosomal disorders such as Turner syndrome (45, XO) and Klinefelter syndrome (47, XXY) are classified as DSDs, even though they may not present with ambiguous genitalia.
Pathophysiology
Normal sexual differentiation begins at approximately 7 weeks of gestation, with the presence of a Y chromosome leading to testicular development. Both male (XY) and female (XX) fetuses initially develop similar reproductive structures, and various genes are involved in this differentiation. Mutations in these genes can result in different forms of DSD. The majority of congenital malformations in genitalia are attributed to:
Androgen Deficiency: Typically affects XY individuals.
Androgen Excess: Common in XX individuals.
Some cases may not have a discernible endocrine cause. Evaluation typically begins with karyotyping and an assessment of adrenal and gonadal function, allowing classification into:
Virilized XX (XX DSD)
Undervirilized XY (XY DSD)
Mixed sex chromosome patterns
Therapeutic Management
Congenital adrenal hyperplasia (CAH) is the most prevalent cause of ambiguous genitalia, which can lead to serious complications, including life-threatening adrenal insufficiency. Timely evaluation is critical. Initial laboratory tests include measuring 17-hydroxyprogesterone and performing a karyotype with an SRY gene probe. Monitoring for signs of adrenal insufficiency (e.g., hypoglycemia, hyponatremia, hyperkalemia) is vital, and appropriate fluid and electrolyte replacement may be necessary. Additional testing, including imaging studies like pelvic and abdominal ultrasound, may be conducted to assess gonadal structures.
Obstructive Uropathy
Definition
Congenital urinary obstruction is characterized by impaired urinary flow that may limit normal renal development. This condition encompasses a wide range of disorders and is one of the most common issues affecting the urinary tract in children. Obstruction may be congenital or acquired, unilateral or bilateral, and can be complete or incomplete.
Pathophysiology
Obstruction can occur at any level within the urinary tract and leads to dilation of the urinary structures above the obstruction. For example:
Ureteropelvic Junction Obstruction (UPJO): The most common site of obstruction, where the ureter meets the renal pelvis.
Posterior Urethral Valves (PUVs): Membranes obstructing the urethra, primarily affecting males.
Congenital Megaureter and Ureterocele: Abnormalities of ureter development that can cause obstruction.
The severity and timing of the obstruction during kidney development can lead to various outcomes, including renal dysplasia and potential dysfunction.
Clinical Manifestations
Symptoms of obstructive uropathy vary based on the location and severity of the obstruction. Common presentations include:
Prenatal Findings: Hydronephrosis detected on fetal ultrasound.
Postnatal Symptoms: Abdominal mass, urinary tract infection, or signs of renal failure. In males, PUVs can lead to respiratory distress or sepsis due to associated oligohydramnios.
Pain and Infection: Conditions like UPJO may present with abdominal or flank pain and nausea. Ureterocele or megaureter may present with urinary tract infections or hematuria.
Obstructive uropathy can also lead to lower urinary tract symptoms, including urinary stream abnormalities, frequency, urgency, and nocturia.
Chapter 24
1. Define the following kidney-related terms: reabsorption, secretion, excretion, peristalsis, efflux, and reflux.
Reabsorption: This is the process by which substances are transported from the renal tubules back into the blood in the surrounding capillaries. Reabsorption allows the body to reclaim water, electrolytes, and essential nutrients from the glomerular filtrate to maintain homeostasis and prevent excessive loss of vital substances.
Secretion: This refers to the transport of substances from the blood into the tubular lumen. Secretion allows the kidneys to eliminate waste products and excess ions that are not required by the body, thus helping regulate body fluid composition.
Excretion: Excretion is the elimination of substances from the body, primarily through urine. It includes the removal of waste products, excess water, and solutes that are not reabsorbed during renal processes.
Peristalsis: Peristalsis is a series of wave-like muscle contractions that move contents through the digestive tract and other tubular structures in the body. In the context of the kidneys, peristalsis refers to the muscular movements in the ureters that propel urine from the renal pelvis to the urinary bladder.
Efflux: Efflux is the process of moving substances out of a compartment or structure. In the renal context, it typically refers to the movement of urine from the kidneys through the ureters to the bladder.
Reflux: Reflux is the backward flow of fluid, in this case, urine moving from the bladder back into the ureters or kidneys. This can occur due to anatomical defects or increased pressure within the bladder during micturition, which can lead to potential complications.
2. Review the structural and functional unit of the kidney.
The nephron is the structural and functional unit of the kidney, responsible for the formation of urine and the regulation of water, electrolytes, and waste products. Each nephron consists of several components:
Bowman Capsule: A cup-like sac that encases a tuft of capillaries (glomerulus). It is the site where filtration of blood begins, allowing water, ions, and small molecules to pass into the filtrate while retaining larger molecules like proteins.
Glomerulus: A network of capillaries within the Bowman capsule where blood filtration occurs. The glomerular capillaries are highly permeable and allow for the filtration of plasma while retaining cells and large proteins.
Proximal Convoluted Tubule (PCT): The segment following the Bowman capsule where significant reabsorption of water, ions (especially sodium), and nutrients (such as glucose and amino acids) occurs. This segment reabsorbs about 65-70% of the filtered sodium and water.
Loop of Henle: A U-shaped section consisting of a descending limb and an ascending limb. The descending limb is permeable to water but not to solutes, while the ascending limb is impermeable to water and actively transports sodium and chloride ions out, contributing to the concentration gradient in the medulla that aids in urine concentration.
Distal Convoluted Tubule (DCT): The segment that further modifies the filtrate. It reabsorbs sodium, chloride, and calcium, and is also involved in the secretion of potassium and hydrogen ions. The DCT is regulated by hormones such as aldosterone and parathyroid hormone.
Collecting Duct: The final segment of the nephron where the final concentration of urine occurs. The collecting duct reabsorbs water under the influence of antidiuretic hormone (ADH) and is responsible for the final composition and volume of urine that is excreted.
The nephron is surrounded by a rich blood supply that includes the afferent arterioles, which supply blood to the glomeruli, and the efferent arterioles, which carry blood away. The efferent arterioles further branch into the peritubular capillaries and vasa recta, which are involved in the reabsorption and secretion processes that take place along the nephron. Each nephron's design facilitates efficient filtration, selective reabsorption, and waste secretion, making the kidneys highly effective in maintaining homeostasis in body fluid composition.
3. List signs and symptoms of urinary tract disorders or diseases in children of different ages.
Neonatal Period (Birth to 1 Month)
Poor feeding
Vomiting
Failure to gain weight
Rapid respiration (often due to acidosis)
Respiratory distress
Spontaneous pneumothorax or pneumomediastinum
Frequent urination
Screaming on urination
Poor urinary stream
Jaundice
Seizures
Dehydration
Other anomalies or stigmata
Enlarged kidneys or bladder
Infancy (1 to 24 Months)
Poor feeding
Vomiting
Failure to gain weight
Excessive thirst
Frequent urination
Straining or screaming on urination
Foul-smelling urine
Pallor
Fever
Persistent diaper rash
Seizures (with or without fever)
Dehydration
Enlarged kidneys or bladder
Childhood (2 to 14 Years)
Poor appetite
Vomiting
Growth failure
Excessive thirst
Enuresis (bedwetting), incontinence, or frequent urination
Painful urination (dysuria)
Swelling of the face (periorbital edema)
Seizures
Pallor
Fatigue
Blood in urine (hematuria)
Abdominal or back pain
Edema (swelling)
Hypertension (high blood pressure)
Tetany (muscle cramps or spasms)
4. Identify nursing responsibilities in the assessment of renal disorders and diseases in children.
1. Ongoing Assessments
Weight and Height Monitoring: Accurate measurement and recording of the child’s weight and height are crucial to assess growth and fluid status.
Intake and Output (I&O): Careful tracking of fluid intake and urine output to identify potential imbalances or renal dysfunction.
Blood Pressure Measurement: Regular monitoring of blood pressure, as hypertension can be both a consequence and a contributing factor to renal disease.
2. Observation and Assessment
Clinical Manifestations: Observe for general and specific symptoms of renal dysfunction, such as changes in urine characteristics, signs of edema, pallor, or hypertension.
Symptom Recognition: Be vigilant for symptoms that may indicate complications or exacerbation of renal conditions, such as fever, vomiting, or abdominal pain.
3. Preparation for Diagnostic Tests
Patient and Family Education: Prepare infants, children, and their families for diagnostic tests and procedures, providing information about what to expect and how to prepare.
Urine and Blood Specimen Collection: Assist in the collection of urine and blood specimens, ensuring correct procedures are followed for accurate results.
4. Urine Analysis and Characteristics
Observation of Urine Characteristics: Monitor and record characteristics of urine, including color, clarity, odor, and volume.
Perform Urine Tests: Conduct tests on urine specimens, such as specific gravity, presence of protein, blood, glucose, and ketones.
5. Collaboration with Healthcare Team
Assist with Diagnostic Tests: Support and assist in more complex diagnostic tests, such as imaging studies or renal biopsies.
Familiarity with Laboratory Tests: Understand and interpret significant laboratory tests relevant to renal function and their implications for care.
6. Medication Safety and Alerts
Drug Alerts: Be aware of contraindications and risks associated with medications and interventions. For example, avoid using Fleet enemas in children with acute or chronic renal failure due to the risk of hyperphosphatemia and ensure thorough investigation before implementing such requests.
7. Monitoring for Complications
Identify At-Risk Patients: Recognize children at risk for renal complications (e.g., those in shock, postoperative patients) and prioritize assessments accordingly.
5. Outline the factors that contribute to the development of urinary tract infections (UTIs) in infants and children.
1. Microbial Factors
Common Uropathogens: Escherichia coli is the most prevalent cause of UTIs, especially in females. Other gram-negative bacteria associated with UTIs include Proteus mirabilis, Pseudomonas aeruginosa, Klebsiella, and Enterobacter. Gram-positive bacteria like Enterococcus and Staphylococcus saprophyticus can also cause UTIs, while viruses and fungi are rare causes in children.
Virulence Factors: These uropathogens possess specialized virulence factors that enable them to attach to and ascend the urinary tract. For instance, E. coli has pili and fimbriae that allow it to bind to the periurethral mucosa and move upward into the bladder.
2. Anatomical and Physiological Factors
Shorter Urethra in Females: Females have a shorter urethra, which provides an easier path for bacteria to reach the bladder. This anatomical feature, along with the closer proximity to the gastrointestinal tract, increases the risk of bacterial migration.
Uncircumcised Males: The presence of a foreskin in males is associated with a higher risk of UTI due to increased periurethral bacterial colonization. Studies show that uncircumcised infants have a significantly higher incidence of UTI compared to circumcised males.
Urinary Stasis: The most significant host factor for UTI development is urinary stasis. When the bladder is not completely emptied, it allows bacteria to persist and multiply. Causes of urinary stasis include:
Anatomic Abnormalities: Conditions like vesicoureteral reflux (VUR), where urine flows backward from the bladder to the ureters, contribute to urinary retention.
Functional Issues: Conditions like neurogenic bladder and dysfunctional voiding lead to incomplete bladder emptying, increasing UTI risk.
3. Functional and Behavioral Factors
Bowel-Bladder Dysfunction (BBD): BBD is characterized by symptoms like urinary frequency, urgency, and constipation, all of which increase UTI risk by promoting urinary stasis. It often occurs in children with pelvic floor dysfunction or those with abnormal voiding habits developed during or after toilet training.
Holding Back Urine: Children may voluntarily hold back urine, leading to bladder overdistention and incomplete emptying. This creates an environment favorable for bacterial growth.
Dysfunctional Voiding: Some children contract the urethral sphincter while urinating, leading to urinary stasis and increased risk of infection.
4. Extrinsic Factors
Constipation: A full rectum can press against the bladder, causing incomplete emptying and urinary stasis. Treating constipation is essential for reducing UTI recurrence.
Catheter Use: Indwelling catheters can introduce bacteria into the urinary tract, increasing infection risk, particularly with prolonged use.
Antibiotic Use: Antibiotics disrupt normal perineal flora, which can make it easier for pathogenic bacteria to colonize the urinary tract.
5. Environmental and Hygienic Factors
Poor Hygiene: Improper cleaning practices, especially in young children, can introduce bacteria into the urethra, increasing the risk of infection.
Irritants: Bubble baths, essential oils, and some shampoos can irritate the urethra, causing frequent and painful urination.
Hot Tubs and Whirlpools: The use of hot tubs or whirlpool baths is associated with an increased risk of UTIs due to bacterial exposure.
Sexual Activity (in Older Children): Sexual intercourse may introduce bacteria into the urinary tract, leading to transient bacteriuria and increased UTI risk in females.
6. Define the following terms used to classify UTIs and urinary inflammation: bacteriuria, asymptomatic bacteriuria, symptomatic bacteriuria, recurrent UTI, persistent UTI, febrile UTI, cystitis, urethritis, pyelonephritis, and urosepsis.
Bacteriuria: Presence of bacteria in the urine.
Asymptomatic Bacteriuria: Significant bacteriuria (typically >100,000 colony-forming units per mm³) without clinical symptoms of infection.
Symptomatic Bacteriuria: Bacteriuria accompanied by symptoms of a urinary infection, such as dysuria (painful urination), suprapubic discomfort, hematuria (blood in urine), or fever.
Recurrent Urinary Tract Infection (UTI): Repeated episodes of bacteriuria or symptomatic UTI.
Persistent UTI: Continued presence of bacteriuria despite appropriate antibiotic treatment.
Febrile UTI: Bacteriuria with accompanying fever and other physical signs of infection; fever often suggests that the infection has reached the kidneys (pyelonephritis).
Cystitis: Inflammation of the bladder.
Urethritis: Inflammation of the urethra.
Pyelonephritis: Inflammation of the upper urinary tract, particularly affecting the kidneys.
Urosepsis: A febrile UTI associated with systemic signs of infection, often confirmed by the presence of urinary pathogens in the bloodstream (positive blood culture).
7. Discuss the clinical manifestations of UTIs in children of various ages.
Infants and Toddlers (under 2 years old):
Often present with nonspecific symptoms such as fever, irritability, lethargy, poor feeding, vomiting, and diarrhea.
Newborns may show fever or hypothermia, jaundice, tachypnea, or cyanosis and may appear significantly ill.
Children Over 2 Years:
More likely to exhibit classic UTI symptoms, including enuresis (bedwetting) or daytime incontinence in previously toilet-trained children, fever, foul-smelling urine, increased urination frequency, dysuria (painful urination), and urgency.
Some may report abdominal pain or flank pain (costovertebral angle tenderness).
Additional symptoms may include hematuria (blood in urine) or vomiting.
In younger boys, obstructive symptoms like dribbling of urine, straining, or reduced urine stream force may appear.
Symptoms of Pyelonephritis:
High fever, chills, and flank pain may suggest kidney infection.
Severe abdominal pain and leukocytosis (high white blood cell count) can also indicate pyelonephritis.
Physical examination may reveal flank tenderness as the primary sign.
Older Children and Adolescents:
Lower urinary tract infections (bladder or urethra) generally cause frequency, urgency, and painful urination. The urine may be cloudy or blood-tinged, and fever is either absent or low-grade.
Upper urinary tract infections (kidney involvement) are usually marked by higher fever (>38°C), chills, and flank pain, sometimes combined with symptoms of lower tract infections.
Asymptomatic or Atypical Presentations:
Many children have asymptomatic or atypical presentations, with symptoms that may resemble respiratory or gastrointestinal infections.
Identifying UTIs in these cases is critical to prevent renal scarring, especially in infants and young children.
Dysfunctional Voiding Symptoms :
Includes urinary frequency changes, constipation, straining, urgency, squatting to prevent wetting, and daytime/nighttime wetting.
8. Describe common side effects of urinary antiinfective agents and nursing interventions to minimize these side effects.
DrugSide EffectsNursing Interventions | ||
Trimethoprim-sulfamethoxazole (Bactrim, Septra) | Rash, urticaria, photosensitivity, nausea, bone marrow depression (long-term use) | - Encourage adequate fluid intake to prevent crystalluria. |
Amoxicillin (Amoxil, Polymox, Trimox) | Nausea, vomiting, diarrhea | - Refrigerate the suspension and discard after 14 days. |
Nitrofurantoin (Macrodantin, Furadantin) | Nausea, pneumonitis, or pulmonary fibrosis (long-term use) | - Administer with food or milk to decrease nausea. |
Cephalexin (Keflex) | Nausea, diarrhea | - Administer with food or milk. |
Ceftazidime (Fortaz) | Renal toxicity | - Monitor renal function through lab values, such as BUN and creatinine. |
Gentamicin (Garamycin) | Renal toxicity, ototoxicity | - Monitor renal function closely. |
Additional Care and Interventions for Suspected Pyelonephritis:
Hospital Admission: Infants and young children with suspected pyelonephritis and fever may require hospital admission for intravenous (IV) antibiotics and hydration.
Culture Testing: Blood and urine cultures are typically obtained upon admission and after therapy to monitor infection response.
Nitrofurantoin Use Limitation: Nitrofurantoin is avoided in febrile infants or in cases of suspected pyelonephritis, as it does not reach therapeutic levels in the blood or kidney.
9. Discuss vesicoureteral reflux (VUR) and the International Classification System used to describe the level of reflux into upper urinary tract structures.

Vesicoureteral reflux (VUR) involves the backward flow of urine from the bladder into the ureters and sometimes up to the kidneys. While VUR itself does not directly cause urinary tract infections (UTIs), it increases the risk of febrile UTIs and may contribute to kidney damage if the refluxing urine carries bacteria to the upper urinary tract. The pressure during voiding or filling of the bladder can force urine back into the ureters, filling the renal pelvis and potentially leaving residual urine in the ureter after urination, which increases infection risk.
International Classification System for VUR
The International Classification System categorizes VUR into five grades based on the degree to which urine refluxes into the upper urinary tract structures:
Grade I: Urine refluxes only into the ureter, without reaching the renal pelvis.
Grade II: Reflux reaches the renal pelvis but without any dilation of the ureter or renal pelvis.
Grade III: Reflux reaches the renal pelvis, causing mild to moderate dilation of the ureter and renal pelvis.
Grade IV: Reflux causes moderate dilation of the ureter and renal pelvis with mild blunting of the renal calyces.
Grade V: Severe dilation of the ureter, renal pelvis, and calyces, along with loss of the normal papillary impressions, which suggests more severe kidney involvement.
Types and Causes of VUR
Primary VUR: Caused by a congenital defect at the ureterovesical junction, where the ureter enters the bladder. In this case, the anti-reflux mechanism fails, allowing urine to flow backward.
Secondary VUR: Occurs due to abnormally high bladder pressure from anatomical (e.g., posterior urethral valves) or functional causes (e.g., neurogenic bladder or dysfunctional voiding). Children with neurogenic bladder conditions, such as spina bifida, are particularly susceptible to secondary VUR.
10. Discuss the therapeutic management and nursing care indications for children with VUR.
Therapeutic Management of VUR
Nonoperative Therapy:
Most cases of vesicoureteral reflux (VUR) resolve spontaneously over time, especially in younger children, lower grades of reflux, unilateral reflux, and those diagnosed early (under 1 year). Higher grades of VUR (IV and V) have lower spontaneous resolution rates.
Continuous Antibiotic Prophylaxis (CAP): Used to prevent bacterial multiplication in the urine, CAP involves giving antibiotics such as trimethoprim-sulfamethoxazole, trimethoprim, or nitrofurantoin, usually at bedtime. Amoxicillin may be used in infants under 2 months but is avoided in older children to reduce antibiotic resistance.
CAP is usually continued until VUR resolves. However, treatment is increasingly individualized, considering factors like the child’s toilet training, voiding habits, and family preferences. Parents and caregivers must closely monitor the child for any signs of UTI or unexplained fever, as breakthrough infections can still occur despite CAP.
Surgical Management:
Surgical intervention may be necessary in cases where reflux is unlikely to resolve and poses a risk of renal damage (e.g., Grade V with renal scarring, high-grade VUR in children older than 6, or recurrent UTIs despite CAP).
Surgical Options:
Open Surgical Correction: Involves reimplanting the ureter to correct the abnormal insertion into the bladder. This procedure requires a short hospital stay.
Endoscopic Correction: A minimally invasive procedure that injects a bulking agent (dextranomer/hyaluronic acid) beneath the ureterovesical junction mucosa to alter the ureter’s angle and reduce reflux. This outpatient procedure is less invasive but may be less durable over time, with some children experiencing recurrence of reflux.
Postoperative Monitoring:
Renal ultrasonography is performed one month after surgery to monitor for ureteral obstruction and confirm surgical success.
Nursing Care Management for Children with VUR
Promoting Compliance with Medical Therapy:
Educate parents and older children on the importance of adhering to the prescribed antibiotic regimen to prevent UTIs.
Provide guidance on methods to encourage medication compliance, especially in young children, and emphasize hygiene practices and frequent voiding schedules.
Inform parents about recognizing UTI symptoms and the need for prompt medical attention, as breakthrough infections can occur even with CAP.
Sibling Screening and Family Education:
Since VUR has a familial tendency, nurses should inform parents of the potential risk for siblings. Screening with renal ultrasound or cystography is recommended if there is a family history of UTIs or abnormal findings on ultrasound.
Encourage awareness of VUR symptoms in other children and prompt evaluation if there are signs of UTI.
Preparation and Support for Procedures:
Prepare children appropriately for diagnostic procedures (e.g., renal ultrasound, VCUG) and consider using lidocaine jelly to ease discomfort during catheterization.
Ensure that children and parents understand the process and are comfortable with it, as preparation can reduce anxiety and improve cooperation.
Follow-Up and Support:
Follow-up care is essential, especially in children with high-grade VUR or frequent UTIs. Regular monitoring of renal health and growth is crucial to prevent long-term complications such as renal scarring or chronic kidney disease.
11. Identify the etiology and common signs and symptoms of acute glomerulonephritis (AGN) as a primary event and as a manifestation of a systemic disorder.
Etiology:
Primary Event: AGN can develop primarily within the kidney due to an immune response or infection, often as a postinfectious reaction to certain bacteria (e.g., group A β-hemolytic streptococcus) or viruses. The infection triggers immune complex formation, which deposits in the glomeruli and leads to inflammation.
Systemic Disorder: AGN can also manifest as part of a systemic condition, such as lupus nephritis or Henoch-Schönlein purpura, where the body’s immune response affects multiple organs, including the kidneys.
Signs and Symptoms:
Oliguria (reduced urine output)
Edema, especially facial/periorbital, spreading to extremities and abdomen
Hypertension due to fluid retention and circulatory congestion
Hematuria (cola or tea-colored urine) indicating blood in the urine
Proteinuria (presence of protein in the urine)
Other general symptoms: irritability, lethargy, anorexia, headache, abdominal discomfort, and in severe cases, dysuria.
12. Discuss the role of poststreptococcal infection in the incidence of acute poststreptococcal glomerulonephritis (APSGN).
Role of Poststreptococcal Infection in Acute Poststreptococcal Glomerulonephritis (APSGN)
APSGN is the most common noninfectious renal disease in children and typically follows a latent period of 1-2 weeks after a streptococcal throat infection or 3-6 weeks after a skin infection like impetigo.
APSGN occurs due to an immune-complex reaction involving antibodies formed in response to streptococcal antigens. These immune complexes become trapped in the glomeruli, causing inflammation and structural changes.
APSGN often appears in early school-age children (3-12 years) and is most prevalent following streptococcal infections in colder months or during late summer and early fall in warmer climates.
13. Describe the major complications of AGN.
Major Complications of AGN
Hypertensive Encephalopathy:
Cause: Severe, acute hypertension can lead to a failure of cerebral autoregulation, causing brain hyperperfusion and cerebral edema.
Signs: Headache, dizziness, vomiting, blurred vision, disorientation, and seizures.
Acute Cardiac Decompensation:
Cause: Hypervolemia, rather than heart failure, increases fluid load on the heart, causing circulatory congestion and potentially pulmonary edema.
Signs: Enlarged heart, increased pulmonary vascular markings, and possibly respiratory symptoms.
Acute Kidney Injury (AKI):
Cause: Severe and prolonged glomerular inflammation may lead to oliguria or anuria.
Signs: Persistent low urine output, increased blood urea nitrogen (BUN) and creatinine, potentially necessitating supportive therapy.
14. Discuss the pathophysiology, clinical manifestations, and therapeutic management for a child with chronic glomerulonephritis.
Pathophysiology of Chronic Glomerulonephritis (CGN)
Chronic glomerulonephritis (CGN) encompasses a group of kidney disorders characterized by long-term inflammation and damage to the glomeruli, which are the filtering units of the kidney. The pathophysiological processes involved in CGN can be multifactorial and include:
Immunologic Mechanisms: CGN is often associated with immune-mediated injury. This may occur through:
Direct Attack: Autoantibodies target kidney tissues, leading to inflammation and damage.
Immune Complex Deposition: Immune complexes formed by antibodies and antigens can deposit in the glomeruli, activating the complement system and causing inflammation.
Secondary Immune Response: Previous kidney injury may result in chronic inflammation and fibrosis as the immune system attempts to repair the damage.
Structural Changes: The chronic inflammation leads to glomerular scarring (sclerosis) and damage, ultimately affecting kidney function. Over time, the glomeruli may lose their ability to filter blood effectively, resulting in nephron loss and renal fibrosis.
Progression to End-Stage Renal Disease (ESRD): The long-term consequences of CGN include progressive renal insufficiency. As nephron damage accumulates, renal function declines, leading to the potential need for dialysis or kidney transplantation.
Clinical Manifestations
The clinical presentation of CGN can vary widely, especially depending on the underlying cause. Early in the disease, symptoms may be minimal or asymptomatic. However, as the disease progresses, the following manifestations may occur:
Proteinuria: This can range from mild to nephrotic syndrome levels, often detectable on routine urinalysis.
Hematuria: Microscopic or gross hematuria may be present.
Hypertension: High blood pressure is common due to fluid overload and renal impairment.
Edema: Swelling may occur, particularly in the face, abdomen, or legs, due to fluid retention.
Signs of Chronic Kidney Disease: These may include fatigue, anemia, and electrolyte imbalances (e.g., hyperkalemia, metabolic acidosis).
Growth Retardation: In children, chronic kidney disease can impact growth and development.
Therapeutic Management
Management of CGN aims to address the underlying cause, control symptoms, and preserve kidney function. Key components include:
Symptomatic Treatment: Early stages may require symptomatic management, focusing on optimizing the child’s health, growth, and quality of life. This includes:
Dietary modifications: Reduced sodium and protein intake may be recommended to manage edema and renal workload.
Fluid management: Careful monitoring of fluid intake to prevent overload.
Pharmacological Interventions:
Corticosteroids: Used in some cases, particularly those associated with nephrotic syndrome or significant inflammation.
Immunosuppressive agents: Cytotoxic medications may be indicated in specific types of CGN.
Antihypertensive Agents: ACE inhibitors or angiotensin II receptor blockers (ARBs) to manage hypertension and protect kidney function.
Erythropoietin: Administered for anemia management in patients with chronic kidney disease.
Monitoring and Follow-Up: Regular monitoring of renal function, electrolytes, and overall health is crucial.
Renal Replacement Therapy: In advanced stages, when renal function is severely compromised, dialysis or kidney transplantation may be necessary.
Nursing Care Management
Nursing care for children with CGN includes:
Education and Support: Providing information about the disease process, treatment options, and the importance of adherence to therapy.
Monitoring: Regular assessment of vital signs, weight, and laboratory values to detect complications early.
Psychosocial Support: Addressing the emotional and developmental needs of the child, encouraging normalcy in daily activities as much as possible.
Coordination of Care: Collaborating with a multidisciplinary team to provide comprehensive care tailored to the child’s needs.
15. Name the three types of nephrotic syndrome and common clinical manifestations, therapeutic management, and nursing care management for children with these conditions.
Nephrotic syndrome in children can be classified into three main types: Minimal Change Nephrotic Syndrome (MCNS), Secondary Nephrotic Syndrome, and Congenital Nephrotic Syndrome (Finnish Type). Below is an overview of each type, common clinical manifestations, therapeutic management, and nursing care management associated with these conditions.
1. Minimal Change Nephrotic Syndrome (MCNS)
Common Clinical Manifestations:
Rapid weight gain due to edema, particularly around the eyes in the morning and progressing to abdominal and lower extremity swelling.
Generalized edema (anasarca) can develop.
Decreased urine output, often dark and frothy.
Possible abdominal pain and diarrhea from intestinal edema.
Pallor, fatigue, and irritability.
Susceptibility to infections, particularly cellulitis and pneumonia.
Skin changes, such as white lines (Muehrcke lines) on nails due to hypoalbuminemia.
Therapeutic Management:
Corticosteroid Therapy: Prednisone is the first-line treatment, with a typical dosage of 60 mg/m²/day for 4-6 weeks, then tapered.
Diuretics: May be used to manage edema, though their effectiveness can be limited.
Supportive Care: Treatment of infections, nutritional support, and monitoring of fluid status.
Immunosuppressive Therapy: For steroid-resistant or frequently relapsing cases, medications like cyclophosphamide or rituximab may be considered.
Nursing Care Management:
Daily monitoring of weight, intake/output, and abdominal girth.
Assessment of edema and skin integrity.
Infection prevention strategies, including hand hygiene and isolation from sick individuals.
Nutritional guidance, including managing dietary sodium and providing small, frequent meals.
Education for parents on recognizing signs of relapse and administering medications.
2. Secondary Nephrotic Syndrome
Common Clinical Manifestations:
Similar to MCNS but with an underlying cause, such as infections, drugs, or systemic diseases (e.g., lupus).
The clinical presentation can vary depending on the associated condition.
Therapeutic Management:
Treat Underlying Cause: Addressing the primary condition (e.g., treating infections, adjusting medications).
Supportive Care: Similar to MCNS, including corticosteroids if indicated.
Nursing Care Management:
Close monitoring for signs of complications related to the underlying disease.
Family education regarding the systemic illness and its management.
Coordination of care across specialties as needed.
3. Congenital Nephrotic Syndrome (Finnish Type)
Common Clinical Manifestations:
Symptoms manifest within the first few days to months of life, including significant proteinuria and edema.
Infants are typically small for gestational age.
Severe and progressive course, often not responsive to standard therapies.
Therapeutic Management:
Supportive Care: IV albumin, nutritional support, dialysis, or kidney transplant may be necessary.
Close monitoring of growth and metabolic status.
Nursing Care Management:
Intensive monitoring of weight and nutritional status.
Education and support for families regarding the prognosis and care needs.
Emotional support for families facing the challenges of congenital conditions.
General Considerations for All Types
Monitoring and Assessment: Daily measurements of vital signs, weight, and fluid status are crucial.
Infection Control: High susceptibility to infections necessitates protective measures.
Family Support: Providing education and emotional support to families is critical, especially during hospitalizations or periods of frequent relapses.
16. Discuss the clinical manifestations and management of the following renal diseases: nephrogenic diabetes insipidus, hemolytic uremic syndrome, familial nephritis (Alport syndrome), and unexplained proteinuria.
Nephrogenic Diabetes Insipidus (NDI)
Clinical Manifestations: NDI is characterized by the kidneys' inability to concentrate urine due to insensitivity to antidiuretic hormone (ADH). In infants, it often presents with:
Polyuria: Large volumes of dilute urine leading to severe dehydration.
Hypernatremia: High sodium levels due to dehydration.
Failure to thrive: Poor growth associated with diminished fluid intake.
Symptoms of dehydration: Vomiting, unexplained fever, irritability, and lethargy.
Management:
Hydration: Ensure adequate fluid intake to compensate for urinary losses.
Dietary modifications: A low-sodium, low-solute diet can help reduce urine output.
Medications: Hydrochlorothiazide and amiloride may be prescribed to enhance proximal tubular reabsorption of sodium and water, thereby reducing urine output. NSAIDs may also help in conjunction with thiazides.
Monitoring and support: Regular follow-up to ensure adequate hydration and manage long-term effects.
Hemolytic Uremic Syndrome (HUS)
Clinical Manifestations: HUS presents as a triad of:
Acute kidney injury (AKI)
Hemolytic anemia: Characterized by pallor and fatigue due to the destruction of red blood cells.
Thrombocytopenia: Low platelet count leading to bleeding tendencies such as bruising and purpura.
Patients often have a prodromal period of gastrointestinal illness (usually diarrhea) followed by rapid onset of the aforementioned symptoms. Severe cases may involve CNS symptoms, seizures, and renal failure.
Management:
Supportive care: Focus on fluid management, correction of electrolyte imbalances, and monitoring of vital signs.
Dialysis: Initiated for those who are anuric or severely oliguric with complications like hypertension or seizures.
Blood transfusions: For severe anemia, done cautiously to prevent fluid overload.
Nutritional support: Enteral or parenteral nutrition may be required if gastrointestinal symptoms are persistent.
Prognosis: With prompt treatment, survival rates are high, but some may experience long-term renal impairment.
Familial Nephritis (Alport Syndrome)
Clinical Manifestations: Alport syndrome is characterized by:
Hematuria: Presenting in infancy, often gross hematuria after respiratory infections.
Progressive renal failure: Typically develops in childhood with varying degrees of proteinuria.
Extrarenal manifestations: Sensorineural hearing loss and ocular abnormalities may also occur.
Management:
Supportive care: Management of blood pressure and proteinuria, often with ACE inhibitors.
Monitoring: Regular renal function tests to track progression.
End-stage renal disease (ESRD) management: Dialysis or kidney transplantation as ultimate interventions.
Genetic counseling: Important for affected families due to hereditary nature.
Unexplained Proteinuria
Clinical Manifestations: Children with unexplained proteinuria often appear healthy but may have:
Transient proteinuria: Commonly occurs during febrile illness, exercise, or dehydration.
Persistent proteinuria: May indicate underlying renal disease.
Orthostatic proteinuria: Common in adolescents, with protein levels higher in the standing position.
Management:
Diagnosis confirmation: Repeat urinalysis, orthostatic testing, and urine culture to exclude renal disease.
Further evaluation: If persistent, may require 24-hour protein excretion tests, renal ultrasound, or other imaging.
Monitoring: Regular follow-up to assess renal function and manage any identified renal pathology.
17. Discuss classifications of renal injury and therapeutic management of renal trauma in children.
18. Compare and contrast the pathophysiology, clinical manifestations, and nursing management of children with acute renal failure and with chronic renal failure (CRF).
Acute Kidney Injury (AKI) Overview
Definition: Acute Kidney Injury (AKI) occurs when the kidneys suddenly lose their ability to regulate urine volume and composition in response to food and fluid intake. Key features include oligoanuria (reduced urine output), azotemia (elevated nitrogenous waste in the blood), acidosis, and electrolyte imbalances. While not common in children, early recognition and treatment are crucial for favorable outcomes.
Etiology
AKI can arise from various clinical conditions, often classified into three categories:
Prerenal Causes:
Description: Related to reduced renal perfusion in a structurally normal kidney.
Common Causes:
Dehydration (from diarrhea or vomiting)
Surgical shock
Trauma (including burns)
Mechanism: Decreased renal perfusion leads to reduced glomerular filtration rate (GFR) and compensatory hormonal responses, potentially causing cortical or tubular necrosis if prolonged.
Intrinsic Renal Causes:
Description: Involves direct damage to the kidney tissues (glomeruli, tubules, or vasculature).
Common Causes:
Glomerular diseases (e.g., glomerulonephritis)
Tubular necrosis (ischemia or nephrotoxins)
Mechanism: Varies depending on the type and extent of damage, leading to varying degrees of renal dysfunction.
Postrenal Causes:
Description: Resulting from obstruction in the urinary tract.
Common Causes: Rare in children but may occur within the first year of life.
Mechanism: Relief of obstruction may restore function; duration of obstruction affects recovery.
Pathophysiology
Glomerular Filtration Rate (GFR): Severely reduced, leading to elevated Blood Urea Nitrogen (BUN) and altered sodium reabsorption.
Hormonal Responses: Increased renin secretion causes afferent arteriolar vasoconstriction, further reducing GFR.
Necrosis Types:
Cortical Necrosis: Severe ischemia or infections; most common in neonates.
Tubular Necrosis: Ischemia and nephrotoxic injuries, resulting in tubular epithelial cell death and possible incomplete recovery.
Clinical Course
Phases of AKI:
Oliguric Phase: Low urine output (<1 ml/kg/hr) with possible anuria.
Diuretic Phase: Abrupt increase in urine output, potentially leading to electrolyte imbalances.
Duration of the oliguric phase can vary significantly based on underlying causes.
Clinical Manifestations
Key Symptoms:
Oliguria or anuria
Edema
Drowsiness
Hypertension
Cardiac arrhythmias due to hyperkalemia
Neurologic symptoms (seizures, altered mental status)
Therapeutic Management
Prevention: Recognize at-risk situations and implement fluid therapy and nephrotoxin precautions.
Fluid and Electrolyte Management:
Restore volume for prerenal causes.
Monitor and restrict electrolytes based on levels and patient needs.
Management of Hyperkalemia:
Monitor serum potassium levels and ECG changes.
Temporary interventions: Calcium gluconate, sodium bicarbonate, insulin and glucose.
Definitive treatment: Sodium polystyrene sulfonate (Kayexalate) or dialysis.
Blood Pressure Control:
Monitor blood pressure every 4–6 hours.
Use antihypertensive medications (e.g., labetalol, sodium nitroprusside) as needed.
Nutritional Support:
Provide high-calorie, low-protein, low-potassium diets.
IV nutritional support if oral intake is insufficient.
Monitoring and Supportive Care:
Intensive monitoring of fluid balance, vital signs, and laboratory values.
Family support and education regarding the child's condition.
Prognosis
Outcomes: Dependent on the cause, severity, and timeliness of management. Mortality rates are generally less than 20%, with favorable outcomes expected in cases of dehydration or nephrotoxins, while HUS or rapidly progressive nephritis may lead to long-term complications.
Nursing Care Management
Focus on monitoring fluid and electrolyte balance, preventing complications, and providing family support.
Prepare for potential dialysis needs and ensure comprehensive care coordination in intensive settings.
Offer empathetic support to both child and family, addressing emotional and informational needs during treatment.
Chronic Kidney Disease (CKD) is a condition characterized by the progressive loss of kidney function over time, often leading to end-stage renal disease (ESRD), where renal replacement therapy, such as dialysis or transplantation, becomes necessary. The following details outline various aspects of CKD, including etiology, pathophysiology, clinical manifestations, diagnostic evaluation, and therapeutic management.
Etiology
The causes of CKD vary by age. In children under 5 years, the most common causes include:
Congenital renal and urinary tract malformations (e.g., renal hypoplasia, dysplasia, obstructive uropathy).
Vesicoureteral reflux (VUR).
For children aged 5 to 15 years, glomerular and hereditary renal diseases become more prevalent, including:
Chronic pyelonephritis.
Chronic glomerulonephritis (CGN).
Hereditary conditions such as Alport syndrome and congenital nephrotic syndrome.
Renal vascular disorders, although they are less common.
Pathophysiology
In the early stages of CKD, children often remain asymptomatic despite some biochemical changes. As nephron damage progresses:
The surviving nephrons undergo hypertrophy to maintain fluid and electrolyte balance.
As nephrons continue to be destroyed, uremic symptoms develop, marked by the retention of waste products, water, sodium, and imbalances in electrolytes and acids.
Key biochemical disturbances include:
Waste Product Retention: Elevated serum creatinine and BUN indicate declining kidney function.
Water and Sodium Retention: Resulting in edema and hypertension, especially in ESRD.
Hyperkalemia: Typically occurs in later stages due to the kidneys' inability to excrete potassium.
Metabolic Acidosis: A hallmark of CKD, due to impaired excretion of hydrogen ions and bicarbonate reabsorption.
Calcium and Phosphorus Disturbances: Can lead to renal osteodystrophy due to complex alterations in mineral metabolism.
Clinical Manifestations
Early signs of CKD may include:
Fatigue and decreased energy.
Pallor due to anemia.
Hypertension.
Growth impairment, observable on growth charts.
As the disease progresses, additional symptoms can manifest, including:
Decreased appetite and altered activity levels.
Urinary output changes (e.g., enuresis).
Symptoms of uremia, such as nausea, vomiting, and itching.
Neurologic symptoms like confusion or seizures may occur in advanced stages.
Diagnostic Evaluation
Diagnosis typically involves:
Clinical Assessment: Identification of symptoms and history of renal disease.
Laboratory Tests: Assessment of renal function through serum creatinine and BUN levels, urine analysis, and imaging studies as needed.
GFR Classification: Determining the stage of CKD based on the glomerular filtration rate (GFR).
Therapeutic Management
Management goals include maximizing renal function, maintaining fluid and electrolyte balance, treating systemic complications, and promoting normal development and quality of life. Key components include:
Activity
Encourage unrestricted activity tailored to the child's stamina.
Facilitate school attendance and home tutoring if necessary.
Diet
Tailored to provide adequate calories and protein while minimizing renal excretory demands.
May involve restrictions on protein, phosphorus, and sodium based on disease stage and symptoms.
Monitoring and supplementation of vitamins and minerals (excluding excess fat-soluble vitamins).
Osteodystrophy Management
Address calcium and phosphorus imbalances through diet, phosphorus-binding agents, and vitamin D supplementation.
Acidosis Treatment
Alkalizing agents like sodium bicarbonate to correct metabolic acidosis.
Anemia Management
Erythropoietin therapy to stimulate red blood cell production, coupled with iron supplementation.
Hypertension Management
Lifestyle modifications with dietary sodium restriction, fluid management, and pharmacological treatment as needed.
Growth and Development Support
Focus on nutrition, correction of imbalances, and potentially growth hormone therapy.
Monitoring for Complications
Regular assessments for intercurrent infections and other complications, adjusting medication dosages based on renal function.
Share
You said:
Discuss the clinical manifestations and management of the following renal diseases: nephrogenic diabetes insipidus, hemolytic uremic syndrome, familial nephritis (Alport syndrome), and unexplained proteinuria Renal Tubular Disorders Disorders of renal tubular function include a variety of conditions involving one or more abnormalities in specific mechanisms of tubular transport or reabsorption. Glomerular function is normal or mildly impaired. Eventually more widespread kidney destruction with renal failure may occur. In some cases the dysfunction has little, if any, effect on renal function. These disorders may be permanent or transient and may originate as primary defects or arise as a secondary effect of metabolic disease or exogenous toxins. Renal tubular disorders may be congenital (usually displaying characteristic patterns of genetic transmission), appear without evidence of hereditary transmission, or be acquired as a result of known or unknown causes. Unlike the classic manifestations of glomerular diseases, edema and hypertension are absent, and the BUN level and routine urinalysis are usually normal. Tubular proteinuria may be demonstrated. Manifestations of tubular disorders are primarily metabolic disturbances or deficiencies, such as failure to thrive, metabolic bone disease, or persistent acidosis. The variety of these disorders is extensive, and the incidence is rare. Tubular Function The function of the proximal tubules is the reabsorption of substances from the glomerular filtrate, including sodium, potassium, chloride, bicarbonate, glucose, phosphate, and amino acids. A number of disorders feature impairment of reabsorption of one or more filtrate constituents, and most involve defects in the transport mechanisms for these substances. Impaired tubular reabsorption of any specific substance causes that substance to appear in the urine, sometimes with reduced levels in the blood. Examples include bicarbonate and phosphate. The primary functions of the distal renal tubules are acidification of urine; potassium secretion; and selective and differential reabsorption of sodium, chloride, and water, which determines the final urinary concentration. Because the contribution of the distal tubule to urine composition depends in part on the volume and composition of the filtrate from the proximal tubule, the net contribution of the distal tubule is related to proximal tubular function and glomerular filtration. Renal Tubular Acidosis Renal tubular acidosis (RTA) is a syndrome of sustained metabolic acidosis in which there is impaired reabsorption of bicarbonate or excretion of net hydrogen ion but in which glomerular function is normal. On the basis of underlying pathophysiology, renal tubular acidosis is divided into proximal renal tubular acidosis and distal renal tubular acidosis. Proximal renal tubular acidosis results from a defect in bicarbonate reabsorption, whereas distal renal tubular acidosis results from an inability to establish an adequate gradient of pH between blood and tubular fluid. A number of genetic abnormalities have been identified for all types of primary RTA (Santos, Ordóñez, Claramunt-Taberner, et al., 2015). Proximal Tubular Acidosis (Type II) Impaired bicarbonate reabsorption in the proximal tubule causes proximal tubular acidosis. It may occur as an isolated defect (primary), but more often it appears in association with other proximal tubular disorders (secondary). As a result of a depressed renal threshold, bicarbonate reabsorption in the proximal tubule is incomplete, causing the plasma concentration of bicarbonate to stabilize at a lower level than normal. This results in a hyperchloremic metabolic acidosis. There is no impairment of distal tubular integrity or, in most cases, of the distal acidifying mechanism. A more complex abnormality in the proximal tubules is Fanconi syndrome, in which transport mechanisms are damaged by the accumulation of toxic metabolites or the tubular epithelium is damaged by heavy metals such as lead, cadmium, or platinum. Fanconi syndrome can be part of a number of hereditary diseases, be acquired, or be idiopathic (with a cause that is not identifiable). The major clinical manifestation and presenting symptom of Fanconi syndrome is growth failure. Tachypnea from hyperchloremic metabolic acidosis is also evident. Dehydration, vomiting, episodic fever, nephrolithiasis secondary to hypercalciuria, muscle weakness or paralysis as a result of hypokalemia, and episodes of severe life-threatening acidemia (sometimes triggered by a concurrent infection) may also be seen. The disorder may be transient or permanent. Distal Tubular Acidosis (Type I) Distal tubular acidosis is caused by the kidney's inability to establish a normal pH gradient between tubular cells and tubular contents. The most characteristic feature is the inability to produce a urinary pH below 6.0 despite the presence of severe metabolic acidosis. Distal renal tubular acidosis usually occurs as a primary, isolated defect but may also occur in association with other diseases or disorders (Gomez, Gil-Peña, & Santos, 2016). Most secondary causes are rare. The primary disorder is usually considered to be a hereditary defect with a variable degree of expression and a greater penetrance in females. After the age of 2 years, the child usually has growth failure, often with a history of vomiting, polyuria, dehydration, anorexia, and failure to thrive. Evidence of bone demineralization may be present, along with the occasional formation of renal calculi in older children. The inability to secrete hydrogen ions causes an accumulation of the ions in the body, which soon depletes the available hydrogen buffer and produces a sustained acidosis. Acidosis slows normal somatic growth, and demineralization of bone occurs as bone salts are mobilized to buffer the excessive hydrogen ions. Increased serum levels of both calcium and phosphorus contribute to the development of stones within the renal system. Both sodium and potassium are secreted in larger amounts. Serum potassium levels are depleted as the distal tubules excrete large amounts of potassium ions in an attempt to conserve sodium because hydrogen ions are unable to participate in the exchange. Hyponatremia stimulates increased aldosterone secretion, which further aggravates the hypokalemia. With the depletion of bicarbonate ions, more chloride is reabsorbed in the proximal tubule to create a hyperchloremia.
ChatGPT said:
ChatGPT
Let's break down the clinical manifestations and management of the renal diseases mentioned: nephrogenic diabetes insipidus, hemolytic uremic syndrome, familial nephritis (Alport syndrome), and unexplained proteinuria. Additionally, we'll touch on renal tubular disorders, particularly renal tubular acidosis (RTA), focusing on proximal and distal types.
1. Nephrogenic Diabetes Insipidus
Clinical Manifestations:
Polyuria: Excessive urination (3-20 liters per day) due to the kidney's inability to concentrate urine.
Polydipsia: Increased thirst, often leading to excessive water intake.
Dehydration: Symptoms include dry mouth, thirst, low blood pressure, and electrolyte imbalances.
Nocturia: Frequent urination at night.
Management:
Hydration: Ensuring adequate fluid intake to prevent dehydration.
Dietary changes: A low-protein, low-salt diet can help reduce urine output.
Medications:
Thiazide diuretics can paradoxically reduce urine output in nephrogenic DI.
Non-steroidal anti-inflammatory drugs (NSAIDs) may also reduce urine output.
Underlying cause treatment: Address any underlying conditions, such as chronic kidney disease or medications contributing to DI.
2. Hemolytic Uremic Syndrome (HUS)
Clinical Manifestations:
Triad of symptoms:
Hemolytic anemia: Characterized by fatigue, pallor, and jaundice.
Thrombocytopenia: Low platelet count leading to easy bruising and bleeding.
Acute renal failure: Symptoms may include decreased urine output, edema, and hypertension.
Gastrointestinal symptoms: Often associated with diarrhea (often bloody) due to Shiga toxin-producing E. coli.
Management:
Supportive care:
Fluid management and electrolyte balance are crucial.
Blood transfusions may be necessary for severe anemia.
Dialysis: May be required in cases of acute renal failure.
Avoidance of antibiotics and antidiarrheal medications: These can worsen the condition.
Monitoring: Close observation for complications, including hypertension and neurologic symptoms.
3. Familial Nephritis (Alport Syndrome)
Clinical Manifestations:
Hematuria: Blood in the urine, often an early sign.
Proteinuria: Presence of protein in urine, leading to nephrotic syndrome in advanced cases.
Progressive renal failure: Typically leads to end-stage renal disease by the second or third decade of life.
Ocular and auditory symptoms: Hearing loss and lens dislocation may occur due to mutations in collagen genes.
Management:
Blood pressure control: Use of ACE inhibitors can help slow progression of kidney disease.
Supportive care: Includes dietary modifications and management of symptoms.
Dialysis or transplant: May be required in cases of end-stage renal disease.
Genetic counseling: Important for affected families.
4. Unexplained Proteinuria
Clinical Manifestations:
Often asymptomatic but can lead to:
Edema
Hypertension
Fatigue or malaise if associated with nephrotic syndrome.
Management:
Assessment of underlying causes: Urinalysis, serum creatinine, and blood tests to rule out primary glomerular disease, infections, or systemic diseases (e.g., diabetes, lupus).
Monitor protein levels: Use urine protein-to-creatinine ratio for quantification.
Treat underlying conditions: Adjustments in management based on the identified cause (e.g., diabetes control, hypertension management).
Long-term follow-up: Regular monitoring of kidney function and proteinuria levels.
Renal Tubular Disorders
Renal Tubular Acidosis (RTA)
Proximal RTA (Type II)
Clinical Manifestations:
Metabolic acidosis with a normal anion gap.
Growth failure (especially in children), rickets, or osteomalacia.
Hypokalemia (low potassium).
Management:
Bicarbonate supplementation to correct acidosis.
Treatment of underlying causes, if secondary (e.g., medications or toxins).
Monitoring for bone health and addressing growth issues.
Distal RTA (Type I)
Clinical Manifestations:
Inability to acidify urine (urinary pH > 6.0).
Metabolic acidosis and growth retardation in children.
Risk of kidney stones and bone disease.
Management:
Bicarbonate supplementation to correct acidosis.
Potassium supplementation if hypokalemia occurs.
Monitoring of renal function and electrolytes.
Conclusion
Understanding the clinical manifestations and management strategies for these renal diseases is crucial in effectively diagnosing and treating patients. Regular monitoring and supportive care are vital to managing chronic conditions and preventing complications.
4o mini
You said:
Discuss the clinical manifestations and management of the following renal diseases: nephrogenic diabetes insipidus, hemolytic uremic syndrome, familial nephritis (Alport syndrome), and unexplained proteinuria Renal Tubular Disorders Disorders of renal tubular function include a variety of conditions involving one or more abnormalities in specific mechanisms of tubular transport or reabsorption. Glomerular function is normal or mildly impaired. Eventually more widespread kidney destruction with renal failure may occur. In some cases the dysfunction has little, if any, effect on renal function. These disorders may be permanent or transient and may originate as primary defects or arise as a secondary effect of metabolic disease or exogenous toxins. Renal tubular disorders may be congenital (usually displaying characteristic patterns of genetic transmission), appear without evidence of hereditary transmission, or be acquired as a result of known or unknown causes. Unlike the classic manifestations of glomerular diseases, edema and hypertension are absent, and the BUN level and routine urinalysis are usually normal. Tubular proteinuria may be demonstrated. Manifestations of tubular disorders are primarily metabolic disturbances or deficiencies, such as failure to thrive, metabolic bone disease, or persistent acidosis. The variety of these disorders is extensive, and the incidence is rare. Tubular Function The function of the proximal tubules is the reabsorption of substances from the glomerular filtrate, including sodium, potassium, chloride, bicarbonate, glucose, phosphate, and amino acids. A number of disorders feature impairment of reabsorption of one or more filtrate constituents, and most involve defects in the transport mechanisms for these substances. Impaired tubular reabsorption of any specific substance causes that substance to appear in the urine, sometimes with reduced levels in the blood. Examples include bicarbonate and phosphate. The primary functions of the distal renal tubules are acidification of urine; potassium secretion; and selective and differential reabsorption of sodium, chloride, and water, which determines the final urinary concentration. Because the contribution of the distal tubule to urine composition depends in part on the volume and composition of the filtrate from the proximal tubule, the net contribution of the distal tubule is related to proximal tubular function and glomerular filtration. Renal Tubular Acidosis Renal tubular acidosis (RTA) is a syndrome of sustained metabolic acidosis in which there is impaired reabsorption of bicarbonate or excretion of net hydrogen ion but in which glomerular function is normal. On the basis of underlying pathophysiology, renal tubular acidosis is divided into proximal renal tubular acidosis and distal renal tubular acidosis. Proximal renal tubular acidosis results from a defect in bicarbonate reabsorption, whereas distal renal tubular acidosis results from an inability to establish an adequate gradient of pH between blood and tubular fluid. A number of genetic abnormalities have been identified for all types of primary RTA (Santos, Ordóñez, Claramunt-Taberner, et al., 2015). Proximal Tubular Acidosis (Type II) Impaired bicarbonate reabsorption in the proximal tubule causes proximal tubular acidosis. It may occur as an isolated defect (primary), but more often it appears in association with other proximal tubular disorders (secondary). As a result of a depressed renal threshold, bicarbonate reabsorption in the proximal tubule is incomplete, causing the plasma concentration of bicarbonate to stabilize at a lower level than normal. This results in a hyperchloremic metabolic acidosis. There is no impairment of distal tubular integrity or, in most cases, of the distal acidifying mechanism. A more complex abnormality in the proximal tubules is Fanconi syndrome, in which transport mechanisms are damaged by the accumulation of toxic metabolites or the tubular epithelium is damaged by heavy metals such as lead, cadmium, or platinum. Fanconi syndrome can be part of a number of hereditary diseases, be acquired, or be idiopathic (with a cause that is not identifiable). The major clinical manifestation and presenting symptom of Fanconi syndrome is growth failure. Tachypnea from hyperchloremic metabolic acidosis is also evident. Dehydration, vomiting, episodic fever, nephrolithiasis secondary to hypercalciuria, muscle weakness or paralysis as a result of hypokalemia, and episodes of severe life-threatening acidemia (sometimes triggered by a concurrent infection) may also be seen. The disorder may be transient or permanent. Distal Tubular Acidosis (Type I) Distal tubular acidosis is caused by the kidney's inability to establish a normal pH gradient between tubular cells and tubular contents. The most characteristic feature is the inability to produce a urinary pH below 6.0 despite the presence of severe metabolic acidosis. Distal renal tubular acidosis usually occurs as a primary, isolated defect but may also occur in association with other diseases or disorders (Gomez, Gil-Peña, & Santos, 2016). Most secondary causes are rare. The primary disorder is usually considered to be a hereditary defect with a variable degree of expression and a greater penetrance in females. After the age of 2 years, the child usually has growth failure, often with a history of vomiting, polyuria, dehydration, anorexia, and failure to thrive. Evidence of bone demineralization may be present, along with the occasional formation of renal calculi in older children. The inability to secrete hydrogen ions causes an accumulation of the ions in the body, which soon depletes the available hydrogen buffer and produces a sustained acidosis. Acidosis slows normal somatic growth, and demineralization of bone occurs as bone salts are mobilized to buffer the excessive hydrogen ions. Increased serum levels of both calcium and phosphorus contribute to the development of stones within the renal system. Both sodium and potassium are secreted in larger amounts. Serum potassium levels are depleted as the distal tubules excrete large amounts of potassium ions in an attempt to conserve sodium because hydrogen ions are unable to participate in the exchange. Hyponatremia stimulates increased aldosterone secretion, which further aggravates the hypokalemia. With the depletion of bicarbonate ions, more chloride is reabsorbed in the proximal tubule to create a hyperchloremia. Prognosis. The primary disorder is usually permanent. However, secondary effects on growth and stone formation can be avoided with early diagnosis and therapy. When the disorder occurs as a secondary complication and renal damage is prevented, the prognosis is good (Gil-Peña, Mejía, & Santos, 2014). Therapeutic Management Treatment of both proximal and distal disorders consists of the administration of sufficient bicarbonate or citrate to balance metabolically produced hydrogen ions; to maintain the plasma bicarbonate level within normal range; and to correct associated electrolyte disorders, especially hypokalemia. Proximal disorders require large volumes of bicarbonate to compensate for urinary losses; in distal disorders the alkali required to maintain a normal plasma concentration is low. Most authorities favor a mixture of sodium and potassium bicarbonate (or citrate) to prevent deficiencies of either cation. The citrate solutions (Bicitra, Polycitra, or Shohl solution) are usually more easily tolerated than bicarbonate solutions. Nursing Care Management Nursing goals include recognizing the possibility of renal tubular acidosis in children who fail to thrive or who display other symptoms suggestive of the disorders and referring these children for medical evaluation. Helping parents understand the importance of adhering to the medication plan as a long-term goal is essential. (See Compliance and Administration of Medication, Chapter 22.) Children who must continue the medication indefinitely need to learn the importance of taking the medications as soon as they are old enough to assume responsibility for their own care. Nephrogenic Diabetes Insipidus Nephrogenic diabetes insipidus (NDI) is the major disorder associated with a defect in the ability to concentrate urine. In this disorder the distal tubules and collecting ducts are insensitive to the action of antidiuretic hormone or its exogenous counterpart, vasopressin. Although several inheritance patterns have been identified, more than 90% of patients have an X-linked defect of the vasopressin receptor (Bichet & Bockenhauer, 2016). The disease is more variable in female carriers of the defective gene, who may exhibit only a mild defect in urine-concentrating ability. The differential diagnosis for NDI should include chronic obstructive renal disorders, sickle cell disease, renal tuberculosis, and other renal disorders that may cause high urinary output with failure of the kidney to respond to vasopressin. Clinical Manifestations and Diagnostic Evaluation NDI is manifested in the newborn period by vomiting, unexplained fever, failure to thrive, and severe recurrent dehydration with hypernatremia. The passage of copious amounts of dilute urine, which produces severe dehydration and hypoelectrolytemia, is a serious threat to life during this period and may be responsible for the high incidence of cognitive impairment and motor retardation found in affected persons. Growth retardation is probably related to diminished food intake and poor general health because of uncontrolled polydipsia. Diagnosis is suspected on the basis of the patient and family history and confirmed by a urine osmolality value consistently below that of plasma. Lack of response to vasopressin administration rules out other causes. Therapeutic Management Therapy involves provision of adequate volumes of water to compensate for urinary losses and minimization of urine output through diet and medication. As a result of an insatiable thirst, most of the child's time is spent drinking and voiding, with decreased time for activity and stimulation. These children may go to great lengths to satisfy their thirst. A low-sodium, low-solute diet and the use of hydrochlorothiazide with or without amiloride to increase the reabsorption of sodium and water in the proximal tubule help reduce the amount of tubular fluid delivered to the distal tubules and to diminish the volume of water excreted (Dabrowski, Kadakia, & Zimmerman, 2016). Urinary output may be reduced when nonsteroidal antiinflammatory drugs (NSAIDs) are administered in conjunction with hydrochlorothiazide. Supplemental potassium may be required to prevent hypokalemia as a result of thiazide therapy. Normal growth and a normal life span are possible if the disease is recognized early and treatment is instituted and maintained. Nursing Care Management Nursing goals for children with NDI and their families are to recognize signs of the disorder early and assist them in coping with the long-term inconvenience of the continual thirst and elimination problems. Families need to learn to administer medications and help with diet planning for those on sodium restriction and needing supplemental potassium. The problem of ensuring adequate hydration is lifelong, and families need to adapt to away-from-home fluid needs and avoid activities that contribute to dehydration when fluids may not be available. Genetic counseling is recommended. Miscellaneous Renal Disorders Hemolytic Uremic Syndrome Hemolytic uremic syndrome (HUS) is an acute renal disease characterized by a triad of manifestations: AKI, hemolytic anemia, and thrombocytopenia. HUS occurs primarily in infants and small children between the ages of 6 months and 3 years. It has been recognized predominantly in Caucasians and, although it occurs worldwide, is more prevalent in South Africa, Argentina, and the west coasts of North and South America. HUS represents one of the main causes of AKI in early childhood. Etiology Diarrhea-positive (D+) HUS accounts for more than 90% of cases and is caused by ingestion of Shiga toxin producing Escherichia coli. E. coli O157:H7 is the most common pathogen, although other serotypes have also been reported (Karpman, Loos, Tati, et al., 2016). Occurrences tend to occur in scattered outbreaks and have been traced to undercooked meat, especially ground beef; unpasteurized apple juice; alfalfa sprouts; and public pools. Diarrhea negative (D−) or atypical HUS may be due to a number of causes, including nonenteric infections, disturbances in the complement system, malignancies, or genetic disorders (Loirat, Fakhouri, Ariceta, et al., 2016). Pathophysiology The primary site of injury appears to be the endothelial lining of the small glomerular arterioles, but other organs and tissues may be involved (e.g., the liver, brain, heart, pancreatic islet cells, and muscles). The endothelium becomes swollen and occluded with the deposition of platelets and fibrin clots (intravascular coagulation). Red blood cells are damaged as they move through the partially occluded blood vessels. The spleen removes these fragmented red blood cells, causing acute hemolytic anemia. Fibrinolytic action on the precipitated fibrin causes these fibrin-split products to appear in the serum and urine. The characteristic thrombocytopenia is produced by the platelet aggregation within damaged blood vessels or the damage and removal of platelets. Clinical Manifestations The disease occurs after a prodromal period, during which there is an episode of diarrhea and vomiting. Less often the preceding illness is an upper respiratory tract infection or, occasionally, varicella, measles, or a UTI. The hemolytic process persists for several days to 2 weeks. During this time the child is anorexic, irritable, and lethargic. There is marked and rapid onset of pallor accompanied by hemorrhagic manifestations such as bruising, purpura, or rectal bleeding. Severely affected patients are anuric and often hypertensive. Seizures and stupor suggest central nervous system involvement, and there may be signs of acute heart failure. Mild cases demonstrate anemia, thrombocytopenia, and azotemia; urinary output may be reduced or increased. Diagnostic Evaluation The triad of anemia, thrombocytopenia, and renal failure is sufficient for diagnosis. Proteinuria, hematuria, and urinary casts are evidence of renal involvement; BUN and serum creatinine levels are elevated. Low hemoglobin and hematocrit and a high reticulocyte count confirm the hemolytic nature of the anemia. Therapeutic Management Treatment is symptomatic and directed toward control of the complications and hematologic manifestations of renal failure. The initial supportive measures for most children are those used in managing renal failure: fluid replacement (calculated with great care), treatment of hypertension, and correction of acidosis and electrolyte disorders (Karpman, Loos, Tati, et al., 2016). The most consistently effective treatment is early hemodialysis, PD, or continuous hemofiltration, which is instituted in any child who has been anuric for 24 hours or who demonstrates oliguria with uremia or hypertension and seizures. Blood transfusions with fresh, washed packed cells are administered for severe anemia but are used with caution to prevent circulatory overload from added volume. Once vomiting and diarrhea have resolved, the child is restarted on enteral nutrition. Sometimes parenteral nutrition is required for children with severe, persistent colitis and for those in whom tissue catabolism is marked. There is no substantial evidence that heparin, corticosteroids, or fibrinolytic agents are beneficial, and in some instances they may aggravate the condition. Ecluzimab, a monoclonal antibody, has shown promise in treating and minimizing recurrences of D-HUS (Loirat, Fakhouri, Ariceta, et al., 2016). Prognosis. With prompt treatment the survival rate of D+ HUS is approximately 95% (Mody, Gu, Griffin, et al., 2015), but residual renal impairment ranges from 10% to 50%. Death is usually caused by residual renal impairment or central nervous system injury. D− HUS has a less favorable outcome, varying according to cause (Durkan, Kim, Craig, et al., 2016). Nursing Care Management Nursing care is the same as that provided in AKI and, for children with continued impairment, includes management of chronic disease. Because of the sudden and life-threatening nature of the disorder in a previously well child, parents are often ill prepared for the impact of hospitalization and treatment. Therefore support and understanding are especially important aspects of care. Nursing Alert To prevent infection from contaminated meat, the internal temperature of the food, such as hamburger, should be at least 74°C (165°F). Cooking the ground beef until no pink color is seen may not be sufficient to kill the bacteria. Therefore a meat thermometer is needed to ensure a safe product. Discourage parents from giving children unpasteurized apple juice and unwashed raw vegetables. Also discourage the use of antimotility drugs for diarrhea. Familial Nephritis (Alport Syndrome) Alport syndrome (AS) is a hereditary disease characterized by high-tone sensorineural deafness, ocular disorders, and chronic kidney disease caused by mutations in type IV collagen. Most people with AS have the X-linked form of the condition. Less common are autosomal recessive and dominant forms. Hematuria presents during infancy in affected boys. Gross hematuria may be associated with acute respiratory tract infections. Proteinuria and progressive renal failure begin in childhood. The progression rate to end-stage kidney disease depends on the form of AS. Females may have only microhematuria or progress to ESRD, again depending on the form of the condition they have (Savige, Colville, Rheault, et al., 2016). Treatment is symptomatic and supportive. Dialysis and kidney transplantation are ultimate therapeutic measures for ESRD. Hearing loss and ocular disorders should receive appropriate attention, and families should be counseled regarding the genetic implications of the disease. Unexplained Proteinuria Often apparently healthy children with no suggestion of renal disease demonstrate proteinuria on routine urinalysis. The percentage of children with unexplained proteinuria ranges from 1% at 6 years of age to 11% at puberty, reaching a maximum prevalence at age 13 in girls and age 16 in boys. Unexplained proteinuria can be categorized as transient (inconstant), persistent, or orthostatic or postural. Transient proteinuria is a common finding with no known cause but sometimes increases with febrile illness, exercise, or dehydration. Persistent proteinuria usually signifies renal disease. Orthostatic proteinuria is seen in 3% to 5% of adolescents and young adults; although proteinuria is evident in both the recumbent and the erect position, it is quantitatively greater in the erect position. The cause is unknown, but minor glomerular changes occur in many instances. The condition is benign and generally resolves over time. In cases of unexplained proteinuria, it is important to confirm or exclude renal disease with appropriate diagnostic tests. Repeated examination for proteinuria, an orthostatic test, urine culture, and (if proteinuria is persistent) more definitive tests—including 24-hour protein excretion, renal ultrasound, and renal scan—are indicated.
ChatGPT said:
ChatGPT
Here's a discussion on the clinical manifestations and management of nephrogenic diabetes insipidus (NDI), hemolytic uremic syndrome (HUS), familial nephritis (Alport syndrome), and unexplained proteinuria, focusing on their renal implications:
Nephrogenic Diabetes Insipidus (NDI)
Clinical Manifestations: NDI is characterized by the kidneys' inability to concentrate urine due to insensitivity to antidiuretic hormone (ADH). In infants, it often presents with:
Polyuria: Large volumes of dilute urine leading to severe dehydration.
Hypernatremia: High sodium levels due to dehydration.
Failure to thrive: Poor growth associated with diminished fluid intake.
Symptoms of dehydration: Vomiting, unexplained fever, irritability, and lethargy.
Management:
Hydration: Ensure adequate fluid intake to compensate for urinary losses.
Dietary modifications: A low-sodium, low-solute diet can help reduce urine output.
Medications: Hydrochlorothiazide and amiloride may be prescribed to enhance proximal tubular reabsorption of sodium and water, thereby reducing urine output. NSAIDs may also help in conjunction with thiazides.
Monitoring and support: Regular follow-up to ensure adequate hydration and manage long-term effects.
Hemolytic Uremic Syndrome (HUS)
Clinical Manifestations: HUS presents as a triad of:
Acute kidney injury (AKI)
Hemolytic anemia: Characterized by pallor and fatigue due to the destruction of red blood cells.
Thrombocytopenia: Low platelet count leading to bleeding tendencies such as bruising and purpura.
Patients often have a prodromal period of gastrointestinal illness (usually diarrhea) followed by rapid onset of the aforementioned symptoms. Severe cases may involve CNS symptoms, seizures, and renal failure.
Management:
Supportive care: Focus on fluid management, correction of electrolyte imbalances, and monitoring of vital signs.
Dialysis: Initiated for those who are anuric or severely oliguric with complications like hypertension or seizures.
Blood transfusions: For severe anemia, done cautiously to prevent fluid overload.
Nutritional support: Enteral or parenteral nutrition may be required if gastrointestinal symptoms are persistent.
Prognosis: With prompt treatment, survival rates are high, but some may experience long-term renal impairment.
Familial Nephritis (Alport Syndrome)
Clinical Manifestations: Alport syndrome is characterized by:
Hematuria: Presenting in infancy, often gross hematuria after respiratory infections.
Progressive renal failure: Typically develops in childhood with varying degrees of proteinuria.
Extrarenal manifestations: Sensorineural hearing loss and ocular abnormalities may also occur.
Management:
Supportive care: Management of blood pressure and proteinuria, often with ACE inhibitors.
Monitoring: Regular renal function tests to track progression.
End-stage renal disease (ESRD) management: Dialysis or kidney transplantation as ultimate interventions.
Genetic counseling: Important for affected families due to hereditary nature.
Unexplained Proteinuria
Clinical Manifestations: Children with unexplained proteinuria often appear healthy but may have:
Transient proteinuria: Commonly occurs during febrile illness, exercise, or dehydration.
Persistent proteinuria: May indicate underlying renal disease.
Orthostatic proteinuria: Common in adolescents, with protein levels higher in the standing position.
Management:
Diagnosis confirmation: Repeat urinalysis, orthostatic testing, and urine culture to exclude renal disease.
Further evaluation: If persistent, may require 24-hour protein excretion tests, renal ultrasound, or other imaging.
Monitoring: Regular follow-up to assess renal function and manage any identified renal pathology.
Conclusion
These renal disorders highlight the diverse manifestations and management strategies required for effective patient care. Early recognition and intervention are crucial to prevent complications and improve long-term outcomes in affected individuals.
4o mini
You said:
Discuss classifications of renal injury and therapeutic management of renal trauma in children. Acute Kidney Injury AKI is said to exist when the kidneys suddenly are unable to appropriately regulate the volume and composition of urine in response to food and fluid intake and the needs of the organism. The principal feature is oligoanuria* associated with azotemia, acidosis, and diverse electrolyte disturbances. AKI is not common in childhood. The outcome depends on the cause, associated findings, and prompt recognition and treatment. Etiology AKI can develop as a result of a large number of related or unrelated clinical conditions: poor renal perfusion, acute renal injury, or the final expression of chronic renal disease. The most common cause in children is transient renal failure resulting from dehydration or other causes of poor perfusion that respond to restoration of fluid volume. Causes of AKI are usually classified as prerenal, intrinsic renal, and postrenal. Severe or long-standing prerenal or postrenal causes can produce severe secondary renal damage. Prerenal Causes. Prerenal causes of AKI are most common in children and are related to the reduction of renal perfusion in an anatomically and physiologically normal kidney and collecting system. Dehydration secondary to diarrheal disease or persistent vomiting is the most common cause of prerenal failure in infants and children. Surgical shock and trauma (including burns) are also common causes. Hypovolemia and decreased renal perfusion cause a decreased glomerular filtration rate and stimulate the secretion of renin, aldosterone, and antidiuretic hormone, which further diminish urine flow. Extended and severe hypoperfusion (secondary to procedures such as cardiac surgery) can produce cortical or tubular necrosis. Increasing awareness of the potential for the development of AKI allows for earlier detection and treatment should it occur (Jefferies & Devarajan, 2016). In general, the azotemia that accompanies this type of renal failure is rapidly reversible with prompt attention to expansion of the extracellular fluid volume. Prerenal failure is often difficult to distinguish from tubular or cortical necrosis. Renal artery stenosis, altered peripheral vascular resistance related to sepsis, and hepatorenal syndrome are less common causes. Intrinsic Renal Causes. Intrinsic renal causes of AKI constitute the largest group that requires extended management. These include diseases and nephrotoxic agents that damage the glomeruli, tubules, or renal vasculature. Glomerular disease is the most common cause of glomerular damage, whereas tubular destruction is more often caused by ischemia or nephrotoxins. Vascular damage is an uncommon cause of renal failure in childhood. The type and extent of damage determine the degree and duration of renal insufficiency, and it is difficult to predict in any given case whether acute necrosis will develop. Postrenal Causes. AKI resulting from obstructive uropathy is uncommon in children except during the first year of life. Relief of the obstruction can restore renal function. The degree of recovery depends on the duration of the renal failure. Pathophysiology AKI is usually reversible, but the deviations of physiologic function can be extreme, and mortality in the pediatric age-group is still high. There is severe reduction in the glomerular filtration rate, an elevated BUN level, and decreased tubular reabsorption of sodium from the proximal tubule. Consequently, there is increased concentration of sodium in the distal tubule, which causes stimulation of the renin mechanism. The local action of angiotensin causes vasoconstriction of the afferent arteriole, which further reduces glomerular filtration and prevents urinary losses of sodium. There is a significant reduction in renal blood flow. The pathologic conditions that produce AKI caused by glomerulonephritis, HUS, and other renal disorders are discussed in relation to those disease processes. The necrotic processes within the nephron can be cortical, tubular, or both. Cortical Necrosis. Complete cortical necrosis usually results from severe ischemia, infection, or intravascular coagulation and represents a severe cause of AKI. In the pediatric age-group this occurs most commonly during the neonatal period as a result of hypoxia and shock. When cortical destruction is incomplete, some recovery of renal function may occur. Tubular Necrosis. Damage to the renal tubules can be broadly classified as secondary to renal ischemia and associated with the ingestion or inhalation of substances toxic to the kidneys. Renal tubules are particularly vulnerable to a wide variety of toxic agents that produce vasoconstriction and to focal patches of ischemia that cause a necrosis of the tubular epithelium down to, but not including, the basement membrane. A lesion produced by sustained reduction in renal blood flow also involves the basement membrane, which may become fragmented and ruptured to the extent that the continuity of tubular structure is disrupted. The lesions may affect any segment of the tubules, appearing at irregular intervals along with normal segments throughout the kidney. Reepithelialization in the areas with intact basement membrane heals tubular lesions. Such healing is unable to take place in areas in which the basement membrane has been disrupted; connective tissue grows through the ruptured membrane, thus preventing reestablishment of tubular integrity. Individual cells within the nephron, but not the entire nephron, are capable of regeneration. Clinical Course. The clinical course of the child with AKI is variable and depends on the cause. In reversible AKI there is a period of severe oliguria, or a low-output phase, followed by an abrupt onset of diuresis, or a high-output phase; this phase is followed by a gradual return to, or toward, normal urine volumes. The length of the oliguric phase in older children and adolescents is 10 to 14 days but is highly variable at all ages depending on the cause of the AKI. The onset of the diuretic phase appears unexpectedly, and over several days it proceeds in stepwise fashion from very low to above-normal urine volumes. During the oliguric phase, manifestations of uremia are present but may also be accompanied by other clinical disorders that make assessment difficult, such as infection, anoxia, and shock. Clinical Manifestations In many instances of AKI the infant or child is already critically ill with the precipitating disorder, and the explanation for development of oliguria may or may not be readily apparent. The underlying illness often overshadows the renal failure and often assumes the priority of care (e.g., the patient who is in shock from endotoxemia, the infant who is severely dehydrated from gastroenteritis, or a child who is subject to seizures as a result of hypertensive encephalopathy associated with AGN). The prime manifestation of AKI is oliguria, generally a urinary output of less than 1 ml/kg/hr. Anuria (no urinary output in 24 hours) is uncommon, except in obstructive disorders. Other symptoms related to AKI include edema, drowsiness, circulatory congestion, and cardiac arrhythmia from hyperkalemia. Seizures may be caused by hyponatremia or hypocalcemia and tachypnea from metabolic acidosis. With continued oliguria, biochemical abnormalities can develop rapidly, and circulatory and central nervous system manifestations appear. Diagnostic Evaluation When a previously well child develops AKI without obvious cause, a careful history is obtained to reveal symptoms that may be related to glomerulonephritis; obstructive uropathy; or exposure to nephrotoxic chemicals, such as ingestion of heavy metals or inhalation of carbon tetrachloride or other organic solvents or drugs (e.g., methicillin, sulfonamides, NSAIDs, neomycin, polymyxin, and kanamycin). Laboratory data reflect the kidney dysfunction: hyperkalemia, hyponatremia, metabolic acidosis, hypocalcemia, anemia, or azotemia (Table 24.7). TABLE 24.7 Laboratory Findings Associated With Acute Renal Failure Clinical Problem Mechanism Clinical Considerations Azotemia Ongoing protein catabolism Lower rate of production in neonates and persons with depleted protein stores Elevated blood urea nitrogen levels Significantly decreased excretion Increased in situations involving large amounts of necrotic tissue or extravasated blood Elevated plasma creatinine levels Continued production Significantly decreased excretion Production less affected by other factors More sensitive measure of intensity of azotemia Low in neonate because of small muscle mass relative to size Metabolic acidosis Continued endogenous acid production Significantly decreased excretion Depletion of extracellular and intracellular fluid buffers Compensatory hyperventilation Opisthonos Major threat to life Hyponatremia Dilution of extracellular fluid Decreased excretion of water May develop cerebral signs Hyperkalemia Ongoing protein catabolism Decreased excretion compounded by metabolic acidosis Most important electrolyte to be considered in acute renal failure May contribute to cardiac arrhythmia With electrocardiogram changes, major threat to life Loss may be from gastrointestinal tract Hypocalcemia Associated with metabolic acidosis and hyperphosphatemia During alkali therapy, may cause tetany Therapeutic Management The most effective management of AKI is prevention. The development of AKI is a known risk in certain situations. This should be anticipated and recognized, and adequate therapy should be implemented (e.g., fluid therapy for children with hypovolemia in conditions such as dehydration, burns, and hemorrhage). Nephrotoxic drugs should be used with caution or avoided in children with renal disease, and all personnel should be knowledgeable about precautions related to their administration. For example, a generous fluid intake is needed for children receiving antimetabolite drugs and after radiotherapy. The treatment of AKI is directed toward treatment of the underlying cause, management of the complications of renal failure, and provision of supportive therapy within the constraints imposed by the renal failure. Treatment of poor perfusion resulting from dehydration consists of volume restoration as described in the treatment of dehydration. (See Chapter 23.) If oliguria persists after restoration of fluid volume or if the renal failure is caused by intrinsic renal damage, the physiologic and biochemical abnormalities that have resulted from kidney dysfunction must be corrected or controlled. Central venous pressure monitoring is usually implemented. Initially a catheter is inserted to rule out urine retention, to collect available urine for electrolytes and analysis, and to monitor the results of diuretic administration. The catheter may or may not be removed. Some clinicians believe that it serves little purpose during the oliguric phase and predisposes the patient to bladder infections. Others maintain a catheter for hourly urine measurements. The use of mannitol, loop diuretics such as furosemide and other medications in the prevention and treatment of AKI have been studied. The 2012 KDIGO Guidelines on Acute Kidney Injury state that the use of mannitol in the prevention of AKI is not scientifically justified due to inadequate studies. They do not recommend the use of diuretics to prevent or treat AKI except as an aid in the management of volume overload. Fluid and Calories. The amount of exogenous water provided should not exceed the amount needed to maintain zero water balance. It is calculated on the basis of estimated endogenous water formation and losses from sensible (primarily gastrointestinal) and insensible sources. No allotment is calculated for urine as long as oliguria persists. The child with AKI has a tendency to develop water intoxication and hyponatremia, both of which make it difficult to provide calories in sufficient amounts to meet the child's needs and reduce tissue catabolism, metabolic acidosis, hyperkalemia, and uremia. If the child is able to tolerate oral foods, concentrated food sources that are high in carbohydrates and fat but low in protein, potassium, and sodium may be provided. However, many children have functional disturbances of the gastrointestinal tract, such as nausea and vomiting. Therefore the IV route is generally preferred, and nourishment usually consists of essential amino acids or a combination of essential and nonessential amino acids administered by the central venous route. Control of water balance in these patients requires careful monitoring of feedback information, such as accurate intake and output, body weight, and electrolyte measurements. In general, during the oliguric phase, no sodium, chloride, or potassium is given unless there are other large, ongoing losses. Regular measurement of plasma electrolytes, pH, BUN, and creatinine levels is required to assess the adequacy of fluid therapy and to anticipate complications that require specific treatment. Hyperkalemia. An elevated serum potassium level is the most immediate threat to the life of the child with AKI. Potassium ions are not being excreted, while at the same time the release of potassium from cells is accelerated by acidosis, stress, and tissue breakdown in cases associated with internal bleeding or trauma. Because cardiac arrhythmia and cardiac arrest may result, electrocardiograms (ECGs) and serum potassium ion levels are monitored regularly. Hyperkalemia can be minimized and sometimes avoided by eliminating potassium from all food and fluids, by reducing tissue catabolism, and by correcting acidosis. Nursing Alert Any of the following signs of hyperkalemia constitute an emergency and should be reported immediately: • Serum potassium concentrations in excess of 7 mEq/L • Presence of ECG abnormalities, such as loss of P wave, prolonged RS complex, depressed ST segment, tall and tented T waves, bradycardia, or heart block Several measures are available to reduce the serum potassium concentration, and the priority of implementation is usually based on the rapidity with which the measures are effective. Temporary measures that produce a rapid but transient effect are as follows: • Calcium gluconate administered intravenously over 2 to 4 minutes with continuous ECG monitoring, exerts a protective effect on cardiac conduction. • Sodium bicarbonate administered intravenously over 30 to 60 minutes, elevates the serum pH to cause a transient shift of extracellular fluid potassium into the intracellular fluid. However, there is a risk of hypocalcemia, tetany, and fluid overload. • Glucose and insulin administered intravenously, accelerate glycogen synthesis, causing glucose and potassium to move into the cells. Insulin facilitates the entry of glucose into cells. These effects produce only transient protection by redistributing existing potassium stores; they do not remove potassium from the body. However, they provide relief while more definitive but slower-acting measures are being implemented. Potassium can be removed by either of two methods: 1. Administration of a cation exchange resin such as sodium polystyrene sulfonate (Kayexalate), 1 g/kg, administered orally or rectally, to bind potassium and remove it from the body. This requires time to be effective, and a sodium ion is exchanged for each potassium ion. This increased sodium concentration adds to the body fluids, which may contribute to fluid overload, hypertension, and cardiac failure. 2. Dialysis or continuous hemofiltration (see p. 810). Hemodialysis is efficient but requires specialized facilities. PD is simpler and can be carried out in almost any hospital setting. Indications for dialysis in AKI are continued oliguria associated with any of the following: • Severe, persistent acidosis • Inability to reduce serum potassium levels to a safe range with other methods • Clinical uremic syndrome consisting of nausea and vomiting, drowsiness, and progression to coma • Circulatory overload, hypertension, and evidence of cardiac failure The optimal timing for initiation of renal replacement therapy is controversial. One strategy is to institute renal replacement therapy within hours of the diagnosis of severe AKI being made, regardless of other symptoms. Another strategy is to delay renal replacement therapy until any of the previously listed symptoms presents. Differences in mortality have not been demonstrated between the two approaches (Gaudry, Hajage, Schortgen, et al., 2016). Hypertension. Hypertension is a common and serious complication of AKI, and blood pressure determinations are taken at least every 4 to 6 hours to detect it early. The most common cause of hypertension in AKI is overexpansion of the extracellular fluid and plasma volume, together with activation of the renin-angiotensin system. The goal of therapy is to prevent hypertensive encephalopathy and avoid overtaxing the cardiovascular system. When there is a threat of encephalopathy, labetalol (a beta and alpha blocker) may be administered intravenously as bolus infusions or a continuous drip. Sodium nitroprusside may be given but requires close monitoring. For less urgent situations, hydralazine, clonidine, or verapamil may be given intravenously. Oral drugs used for acute hypertension include nifedipine, captopril, minoxidil, hydralazine, propranolol, or furosemide. Other Complications. Other complications that may occur with AKI are anemia, seizures and coma, cardiac failure, and pulmonary edema. Anemia is commonly associated with AKI, but transfusion is not recommended unless the hemoglobin level drops below 6 g/dl. Transfusions consist of fresh, packed red blood cells given slowly to reduce the likelihood of increasing blood volume, hypertension, and hyperkalemia. Seizures occur often when renal failure progresses to uremia and are also related to hypertension, hyponatremia, and hypocalcemia. Treatment is directed toward the specific cause when known. More obscure causes are managed with antiepileptic drugs. Cardiac failure with pulmonary edema is almost always associated with hypervolemia. Treatment is directed toward reduction of fluid volume, with water and sodium restriction and administration of diuretics. Digitalis is ineffective and can be hazardous. Diuretic, or High-Output, Phase. When the output begins to increase, either spontaneously or in response to diuretic therapy, the nurse should monitor the intake of fluid, potassium, and sodium, and provide adequate replacement to prevent depletion and its consequences. In some cases the high-output phase is mild and lasts only a few days; in others enormous amounts of electrolyte-rich urine are passed. Prognosis. The prognosis of AKI depends largely on the nature and severity of the causative factor or precipitating event and the promptness and competence of management. The mortality rate is less than 20%. The outcome is least favorable in children with rapidly progressive nephritis and cortical necrosis. Children in whom AKI is a result of HUS or AGN may recover completely, but residual renal impairment or hypertension is more often the rule. Complete recovery is usually expected in children whose renal failure is a result of dehydration, nephrotoxins, or ischemia. AKI after cardiac surgery has a less favorable prognosis. It is often impossible to assess the extent of recovery for several months. (See Quality Patient Outcomes box.) Quality Patient Outcomes Acute Kidney Injury • Underlying cause of acute kidney injury identified and treated • Water balance maintained • Hypertension controlled • Electrolyte balance maintained • Diet maintains calories while minimizing tissue catabolism, metabolic acidosis, hyperkalemia, and uremia Nursing Care Management Nursing care of the infant or child with AKI involves addressing the underlying cause plus carefully observing and managing the renal status. The major goal is reestablishment of renal function (with emphasis on providing an adequate caloric intake to minimize reduction of protein stores); prevention of complications; and monitoring of fluid balance, laboratory data, and physical manifestations. The probability of dialysis or continuous hemofiltration is high, and the nurse must anticipate the availability of the necessary equipment. Because the child requires intensive observation and often specialized equipment, the usual disposition is admission to an intensive care unit where equipment and trained personnel are available. The major nursing tasks in the care of the infant or child with AKI are monitoring and assessing fluid and electrolyte balance. Limiting fluid intake requires ingenuity on the part of caregivers to cope with the child who is thirsty. One strategy involves rationing the daily intake with small amounts of fluid served in containers that give the impression of larger volumes. Older children who understand the rationale of fluid limits can help determine how their daily ration should be distributed. Meeting nutritional needs is sometimes a problem because the child may be nauseated and because getting the child to eat concentrated foods without fluids may be difficult. When nourishment is provided by the IV route, careful monitoring is essential to prevent fluid overload. This can become a major challenge in the face of nutritional requirements and administration of IV medications. The IV drugs being used may be nephrotoxic, which can require a specified volume of solution for delivery. In some instances blood products must also be delivered. Preventing fluid overload while delivering medications and calories requires concerted collaboration. In addition, nursing measures such as maintaining an optimum thermal environment, reducing any elevation of body temperature, and reducing restlessness and anxiety are used to decrease the rate of tissue catabolism. The nurse must be continually alert for behavior changes that indicate the onset of complications. Infection from reduced resistance, anemia, and general morbidity is a constant threat. Fluid overload and electrolyte disturbances can precipitate cardiovascular complications such as hypertension and cardiac failure. Fluid and electrolyte imbalances, acidosis, and accumulation of nitrogenous waste products can produce neurologic involvement manifested by coma, seizures, or alterations in sensorium. Although children with AKI are usually quite ill and voluntarily diminish their activity, infants may become restless and irritable, and children are often anxious and frightened. Frequent, painful, and stress-producing treatments and tests must be performed. A supportive, empathetic nurse can provide comfort and stability in a threatening and unnatural environment. Family Support. Providing support and reassurance to parents is among the major nursing responsibilities. The seriousness and emergency nature of AKI are stressful to parents, and most feel some degree of guilt regarding the child's condition, especially when the illness is the result of ingestion of a toxic substance, dehydration, or a genetic disease. They need reassurance and an empathetic listener. They also need to be kept informed of the child's progress and provided explanations regarding the therapeutic regimen. The equipment and the child's behavior are sometimes frightening and anxiety provoking. Nurses can do much to help parents comprehend and deal with the stresses of the situation.
ChatGPT said:
ChatGPT
Classifications of Renal Injury in Children
Renal injuries in children can be classified based on the mechanisms that cause the injury and their clinical presentation. The primary classifications include:
Acute Kidney Injury (AKI):
Prerenal AKI: This type occurs due to decreased renal perfusion and is the most common cause in children. Causes include dehydration from diarrhea, vomiting, or surgical shock.
Intrinsic Renal AKI: This occurs from direct damage to the kidney tissues, which can be due to glomerular diseases, acute tubular necrosis from ischemia or nephrotoxins, or vascular issues.
Postrenal AKI: This type is caused by obstructive uropathy, which is less common in children but can occur in the neonatal period.
Chronic Kidney Disease (CKD): While not an acute event, chronic kidney conditions can lead to long-term renal injury, affecting a child’s growth and development.
Traumatic Kidney Injury: Renal injuries due to trauma can range from minor contusions to lacerations and vascular injuries. Common causes in children include falls, sports injuries, or blunt trauma.
Therapeutic Management of Renal Trauma in Children
Management of renal trauma requires a systematic approach, often involving both conservative and surgical interventions. Here are key aspects:
1. Initial Assessment and Stabilization
Evaluate Hemodynamics: Monitor blood pressure, heart rate, and perfusion status.
History and Physical Examination: Obtain details regarding the injury mechanism and assess for signs of renal injury, such as hematuria or abdominal pain.
Imaging Studies: Use ultrasound or CT scans to determine the extent of kidney damage and assess for urinary tract injuries.
2. Conservative Management
Observation: Many renal injuries, particularly minor ones, can be managed conservatively. This involves close monitoring of renal function and vital signs.
Fluid Resuscitation: Address hypovolemia with IV fluids as needed. This is critical if there are signs of shock.
Pain Management: Provide adequate analgesia to manage discomfort associated with the injury.
Avoid Nephrotoxic Agents: Reduce the use of medications that can worsen renal function.
3. Surgical Management
Indications for Surgery: Surgical intervention may be required for significant renal lacerations, vascular injuries, or renal pedicle injuries. Other indications include persistent bleeding or failure to respond to conservative management.
Surgical Techniques: Depending on the injury's severity, procedures may range from nephrectomy (removal of the kidney) to renal repair or arterial embolization to control bleeding.
4. Post-Management Monitoring
Renal Function Monitoring: Regularly check serum creatinine and electrolyte levels to evaluate renal function recovery.
Complication Management: Monitor for complications such as urinary obstruction or hypertension, which may require further intervention.
Family Support and Education: Provide emotional support to the family and educate them about the child's condition, treatment options, and recovery process.
Conclusion
Renal injury in children, particularly AKI, requires prompt recognition and appropriate management to minimize long-term complications. Both conservative and surgical approaches may be utilized depending on the severity and nature of the injury. Close monitoring and supportive care are essential components of managing these pediatric renal injuries effectively.
4o mini
You said:
Acute Kidney Injury AKI is said to exist when the kidneys suddenly are unable to appropriately regulate the volume and composition of urine in response to food and fluid intake and the needs of the organism. The principal feature is oligoanuria* associated with azotemia, acidosis, and diverse electrolyte disturbances. AKI is not common in childhood. The outcome depends on the cause, associated findings, and prompt recognition and treatment. Etiology AKI can develop as a result of a large number of related or unrelated clinical conditions: poor renal perfusion, acute renal injury, or the final expression of chronic renal disease. The most common cause in children is transient renal failure resulting from dehydration or other causes of poor perfusion that respond to restoration of fluid volume. Causes of AKI are usually classified as prerenal, intrinsic renal, and postrenal. Severe or long-standing prerenal or postrenal causes can produce severe secondary renal damage. Prerenal Causes. Prerenal causes of AKI are most common in children and are related to the reduction of renal perfusion in an anatomically and physiologically normal kidney and collecting system. Dehydration secondary to diarrheal disease or persistent vomiting is the most common cause of prerenal failure in infants and children. Surgical shock and trauma (including burns) are also common causes. Hypovolemia and decreased renal perfusion cause a decreased glomerular filtration rate and stimulate the secretion of renin, aldosterone, and antidiuretic hormone, which further diminish urine flow. Extended and severe hypoperfusion (secondary to procedures such as cardiac surgery) can produce cortical or tubular necrosis. Increasing awareness of the potential for the development of AKI allows for earlier detection and treatment should it occur (Jefferies & Devarajan, 2016). In general, the azotemia that accompanies this type of renal failure is rapidly reversible with prompt attention to expansion of the extracellular fluid volume. Prerenal failure is often difficult to distinguish from tubular or cortical necrosis. Renal artery stenosis, altered peripheral vascular resistance related to sepsis, and hepatorenal syndrome are less common causes. Intrinsic Renal Causes. Intrinsic renal causes of AKI constitute the largest group that requires extended management. These include diseases and nephrotoxic agents that damage the glomeruli, tubules, or renal vasculature. Glomerular disease is the most common cause of glomerular damage, whereas tubular destruction is more often caused by ischemia or nephrotoxins. Vascular damage is an uncommon cause of renal failure in childhood. The type and extent of damage determine the degree and duration of renal insufficiency, and it is difficult to predict in any given case whether acute necrosis will develop. Postrenal Causes. AKI resulting from obstructive uropathy is uncommon in children except during the first year of life. Relief of the obstruction can restore renal function. The degree of recovery depends on the duration of the renal failure. Pathophysiology AKI is usually reversible, but the deviations of physiologic function can be extreme, and mortality in the pediatric age-group is still high. There is severe reduction in the glomerular filtration rate, an elevated BUN level, and decreased tubular reabsorption of sodium from the proximal tubule. Consequently, there is increased concentration of sodium in the distal tubule, which causes stimulation of the renin mechanism. The local action of angiotensin causes vasoconstriction of the afferent arteriole, which further reduces glomerular filtration and prevents urinary losses of sodium. There is a significant reduction in renal blood flow. The pathologic conditions that produce AKI caused by glomerulonephritis, HUS, and other renal disorders are discussed in relation to those disease processes. The necrotic processes within the nephron can be cortical, tubular, or both. Cortical Necrosis. Complete cortical necrosis usually results from severe ischemia, infection, or intravascular coagulation and represents a severe cause of AKI. In the pediatric age-group this occurs most commonly during the neonatal period as a result of hypoxia and shock. When cortical destruction is incomplete, some recovery of renal function may occur. Tubular Necrosis. Damage to the renal tubules can be broadly classified as secondary to renal ischemia and associated with the ingestion or inhalation of substances toxic to the kidneys. Renal tubules are particularly vulnerable to a wide variety of toxic agents that produce vasoconstriction and to focal patches of ischemia that cause a necrosis of the tubular epithelium down to, but not including, the basement membrane. A lesion produced by sustained reduction in renal blood flow also involves the basement membrane, which may become fragmented and ruptured to the extent that the continuity of tubular structure is disrupted. The lesions may affect any segment of the tubules, appearing at irregular intervals along with normal segments throughout the kidney. Reepithelialization in the areas with intact basement membrane heals tubular lesions. Such healing is unable to take place in areas in which the basement membrane has been disrupted; connective tissue grows through the ruptured membrane, thus preventing reestablishment of tubular integrity. Individual cells within the nephron, but not the entire nephron, are capable of regeneration. Clinical Course. The clinical course of the child with AKI is variable and depends on the cause. In reversible AKI there is a period of severe oliguria, or a low-output phase, followed by an abrupt onset of diuresis, or a high-output phase; this phase is followed by a gradual return to, or toward, normal urine volumes. The length of the oliguric phase in older children and adolescents is 10 to 14 days but is highly variable at all ages depending on the cause of the AKI. The onset of the diuretic phase appears unexpectedly, and over several days it proceeds in stepwise fashion from very low to above-normal urine volumes. During the oliguric phase, manifestations of uremia are present but may also be accompanied by other clinical disorders that make assessment difficult, such as infection, anoxia, and shock. Clinical Manifestations In many instances of AKI the infant or child is already critically ill with the precipitating disorder, and the explanation for development of oliguria may or may not be readily apparent. The underlying illness often overshadows the renal failure and often assumes the priority of care (e.g., the patient who is in shock from endotoxemia, the infant who is severely dehydrated from gastroenteritis, or a child who is subject to seizures as a result of hypertensive encephalopathy associated with AGN). The prime manifestation of AKI is oliguria, generally a urinary output of less than 1 ml/kg/hr. Anuria (no urinary output in 24 hours) is uncommon, except in obstructive disorders. Other symptoms related to AKI include edema, drowsiness, circulatory congestion, and cardiac arrhythmia from hyperkalemia. Seizures may be caused by hyponatremia or hypocalcemia and tachypnea from metabolic acidosis. With continued oliguria, biochemical abnormalities can develop rapidly, and circulatory and central nervous system manifestations appear. Diagnostic Evaluation When a previously well child develops AKI without obvious cause, a careful history is obtained to reveal symptoms that may be related to glomerulonephritis; obstructive uropathy; or exposure to nephrotoxic chemicals, such as ingestion of heavy metals or inhalation of carbon tetrachloride or other organic solvents or drugs (e.g., methicillin, sulfonamides, NSAIDs, neomycin, polymyxin, and kanamycin). Laboratory data reflect the kidney dysfunction: hyperkalemia, hyponatremia, metabolic acidosis, hypocalcemia, anemia, or azotemia (Table 24.7). TABLE 24.7 Laboratory Findings Associated With Acute Renal Failure Clinical Problem Mechanism Clinical Considerations Azotemia Ongoing protein catabolism Lower rate of production in neonates and persons with depleted protein stores Elevated blood urea nitrogen levels Significantly decreased excretion Increased in situations involving large amounts of necrotic tissue or extravasated blood Elevated plasma creatinine levels Continued production Significantly decreased excretion Production less affected by other factors More sensitive measure of intensity of azotemia Low in neonate because of small muscle mass relative to size Metabolic acidosis Continued endogenous acid production Significantly decreased excretion Depletion of extracellular and intracellular fluid buffers Compensatory hyperventilation Opisthonos Major threat to life Hyponatremia Dilution of extracellular fluid Decreased excretion of water May develop cerebral signs Hyperkalemia Ongoing protein catabolism Decreased excretion compounded by metabolic acidosis Most important electrolyte to be considered in acute renal failure May contribute to cardiac arrhythmia With electrocardiogram changes, major threat to life Loss may be from gastrointestinal tract Hypocalcemia Associated with metabolic acidosis and hyperphosphatemia During alkali therapy, may cause tetany Therapeutic Management The most effective management of AKI is prevention. The development of AKI is a known risk in certain situations. This should be anticipated and recognized, and adequate therapy should be implemented (e.g., fluid therapy for children with hypovolemia in conditions such as dehydration, burns, and hemorrhage). Nephrotoxic drugs should be used with caution or avoided in children with renal disease, and all personnel should be knowledgeable about precautions related to their administration. For example, a generous fluid intake is needed for children receiving antimetabolite drugs and after radiotherapy. The treatment of AKI is directed toward treatment of the underlying cause, management of the complications of renal failure, and provision of supportive therapy within the constraints imposed by the renal failure. Treatment of poor perfusion resulting from dehydration consists of volume restoration as described in the treatment of dehydration. (See Chapter 23.) If oliguria persists after restoration of fluid volume or if the renal failure is caused by intrinsic renal damage, the physiologic and biochemical abnormalities that have resulted from kidney dysfunction must be corrected or controlled. Central venous pressure monitoring is usually implemented. Initially a catheter is inserted to rule out urine retention, to collect available urine for electrolytes and analysis, and to monitor the results of diuretic administration. The catheter may or may not be removed. Some clinicians believe that it serves little purpose during the oliguric phase and predisposes the patient to bladder infections. Others maintain a catheter for hourly urine measurements. The use of mannitol, loop diuretics such as furosemide and other medications in the prevention and treatment of AKI have been studied. The 2012 KDIGO Guidelines on Acute Kidney Injury state that the use of mannitol in the prevention of AKI is not scientifically justified due to inadequate studies. They do not recommend the use of diuretics to prevent or treat AKI except as an aid in the management of volume overload. Fluid and Calories. The amount of exogenous water provided should not exceed the amount needed to maintain zero water balance. It is calculated on the basis of estimated endogenous water formation and losses from sensible (primarily gastrointestinal) and insensible sources. No allotment is calculated for urine as long as oliguria persists. The child with AKI has a tendency to develop water intoxication and hyponatremia, both of which make it difficult to provide calories in sufficient amounts to meet the child's needs and reduce tissue catabolism, metabolic acidosis, hyperkalemia, and uremia. If the child is able to tolerate oral foods, concentrated food sources that are high in carbohydrates and fat but low in protein, potassium, and sodium may be provided. However, many children have functional disturbances of the gastrointestinal tract, such as nausea and vomiting. Therefore the IV route is generally preferred, and nourishment usually consists of essential amino acids or a combination of essential and nonessential amino acids administered by the central venous route. Control of water balance in these patients requires careful monitoring of feedback information, such as accurate intake and output, body weight, and electrolyte measurements. In general, during the oliguric phase, no sodium, chloride, or potassium is given unless there are other large, ongoing losses. Regular measurement of plasma electrolytes, pH, BUN, and creatinine levels is required to assess the adequacy of fluid therapy and to anticipate complications that require specific treatment. Hyperkalemia. An elevated serum potassium level is the most immediate threat to the life of the child with AKI. Potassium ions are not being excreted, while at the same time the release of potassium from cells is accelerated by acidosis, stress, and tissue breakdown in cases associated with internal bleeding or trauma. Because cardiac arrhythmia and cardiac arrest may result, electrocardiograms (ECGs) and serum potassium ion levels are monitored regularly. Hyperkalemia can be minimized and sometimes avoided by eliminating potassium from all food and fluids, by reducing tissue catabolism, and by correcting acidosis. Nursing Alert Any of the following signs of hyperkalemia constitute an emergency and should be reported immediately: • Serum potassium concentrations in excess of 7 mEq/L • Presence of ECG abnormalities, such as loss of P wave, prolonged RS complex, depressed ST segment, tall and tented T waves, bradycardia, or heart block Several measures are available to reduce the serum potassium concentration, and the priority of implementation is usually based on the rapidity with which the measures are effective. Temporary measures that produce a rapid but transient effect are as follows: • Calcium gluconate administered intravenously over 2 to 4 minutes with continuous ECG monitoring, exerts a protective effect on cardiac conduction. • Sodium bicarbonate administered intravenously over 30 to 60 minutes, elevates the serum pH to cause a transient shift of extracellular fluid potassium into the intracellular fluid. However, there is a risk of hypocalcemia, tetany, and fluid overload. • Glucose and insulin administered intravenously, accelerate glycogen synthesis, causing glucose and potassium to move into the cells. Insulin facilitates the entry of glucose into cells. These effects produce only transient protection by redistributing existing potassium stores; they do not remove potassium from the body. However, they provide relief while more definitive but slower-acting measures are being implemented. Potassium can be removed by either of two methods: 1. Administration of a cation exchange resin such as sodium polystyrene sulfonate (Kayexalate), 1 g/kg, administered orally or rectally, to bind potassium and remove it from the body. This requires time to be effective, and a sodium ion is exchanged for each potassium ion. This increased sodium concentration adds to the body fluids, which may contribute to fluid overload, hypertension, and cardiac failure. 2. Dialysis or continuous hemofiltration (see p. 810). Hemodialysis is efficient but requires specialized facilities. PD is simpler and can be carried out in almost any hospital setting. Indications for dialysis in AKI are continued oliguria associated with any of the following: • Severe, persistent acidosis • Inability to reduce serum potassium levels to a safe range with other methods • Clinical uremic syndrome consisting of nausea and vomiting, drowsiness, and progression to coma • Circulatory overload, hypertension, and evidence of cardiac failure The optimal timing for initiation of renal replacement therapy is controversial. One strategy is to institute renal replacement therapy within hours of the diagnosis of severe AKI being made, regardless of other symptoms. Another strategy is to delay renal replacement therapy until any of the previously listed symptoms presents. Differences in mortality have not been demonstrated between the two approaches (Gaudry, Hajage, Schortgen, et al., 2016). Hypertension. Hypertension is a common and serious complication of AKI, and blood pressure determinations are taken at least every 4 to 6 hours to detect it early. The most common cause of hypertension in AKI is overexpansion of the extracellular fluid and plasma volume, together with activation of the renin-angiotensin system. The goal of therapy is to prevent hypertensive encephalopathy and avoid overtaxing the cardiovascular system. When there is a threat of encephalopathy, labetalol (a beta and alpha blocker) may be administered intravenously as bolus infusions or a continuous drip. Sodium nitroprusside may be given but requires close monitoring. For less urgent situations, hydralazine, clonidine, or verapamil may be given intravenously. Oral drugs used for acute hypertension include nifedipine, captopril, minoxidil, hydralazine, propranolol, or furosemide. Other Complications. Other complications that may occur with AKI are anemia, seizures and coma, cardiac failure, and pulmonary edema. Anemia is commonly associated with AKI, but transfusion is not recommended unless the hemoglobin level drops below 6 g/dl. Transfusions consist of fresh, packed red blood cells given slowly to reduce the likelihood of increasing blood volume, hypertension, and hyperkalemia. Seizures occur often when renal failure progresses to uremia and are also related to hypertension, hyponatremia, and hypocalcemia. Treatment is directed toward the specific cause when known. More obscure causes are managed with antiepileptic drugs. Cardiac failure with pulmonary edema is almost always associated with hypervolemia. Treatment is directed toward reduction of fluid volume, with water and sodium restriction and administration of diuretics. Digitalis is ineffective and can be hazardous. Diuretic, or High-Output, Phase. When the output begins to increase, either spontaneously or in response to diuretic therapy, the nurse should monitor the intake of fluid, potassium, and sodium, and provide adequate replacement to prevent depletion and its consequences. In some cases the high-output phase is mild and lasts only a few days; in others enormous amounts of electrolyte-rich urine are passed. Prognosis. The prognosis of AKI depends largely on the nature and severity of the causative factor or precipitating event and the promptness and competence of management. The mortality rate is less than 20%. The outcome is least favorable in children with rapidly progressive nephritis and cortical necrosis. Children in whom AKI is a result of HUS or AGN may recover completely, but residual renal impairment or hypertension is more often the rule. Complete recovery is usually expected in children whose renal failure is a result of dehydration, nephrotoxins, or ischemia. AKI after cardiac surgery has a less favorable prognosis. It is often impossible to assess the extent of recovery for several months. (See Quality Patient Outcomes box.) Quality Patient Outcomes Acute Kidney Injury • Underlying cause of acute kidney injury identified and treated • Water balance maintained • Hypertension controlled • Electrolyte balance maintained • Diet maintains calories while minimizing tissue catabolism, metabolic acidosis, hyperkalemia, and uremia Nursing Care Management Nursing care of the infant or child with AKI involves addressing the underlying cause plus carefully observing and managing the renal status. The major goal is reestablishment of renal function (with emphasis on providing an adequate caloric intake to minimize reduction of protein stores); prevention of complications; and monitoring of fluid balance, laboratory data, and physical manifestations. The probability of dialysis or continuous hemofiltration is high, and the nurse must anticipate the availability of the necessary equipment. Because the child requires intensive observation and often specialized equipment, the usual disposition is admission to an intensive care unit where equipment and trained personnel are available. The major nursing tasks in the care of the infant or child with AKI are monitoring and assessing fluid and electrolyte balance. Limiting fluid intake requires ingenuity on the part of caregivers to cope with the child who is thirsty. One strategy involves rationing the daily intake with small amounts of fluid served in containers that give the impression of larger volumes. Older children who understand the rationale of fluid limits can help determine how their daily ration should be distributed. Meeting nutritional needs is sometimes a problem because the child may be nauseated and because getting the child to eat concentrated foods without fluids may be difficult. When nourishment is provided by the IV route, careful monitoring is essential to prevent fluid overload. This can become a major challenge in the face of nutritional requirements and administration of IV medications. The IV drugs being used may be nephrotoxic, which can require a specified volume of solution for delivery. In some instances blood products must also be delivered. Preventing fluid overload while delivering medications and calories requires concerted collaboration. In addition, nursing measures such as maintaining an optimum thermal environment, reducing any elevation of body temperature, and reducing restlessness and anxiety are used to decrease the rate of tissue catabolism. The nurse must be continually alert for behavior changes that indicate the onset of complications. Infection from reduced resistance, anemia, and general morbidity is a constant threat. Fluid overload and electrolyte disturbances can precipitate cardiovascular complications such as hypertension and cardiac failure. Fluid and electrolyte imbalances, acidosis, and accumulation of nitrogenous waste products can produce neurologic involvement manifested by coma, seizures, or alterations in sensorium. Although children with AKI are usually quite ill and voluntarily diminish their activity, infants may become restless and irritable, and children are often anxious and frightened. Frequent, painful, and stress-producing treatments and tests must be performed. A supportive, empathetic nurse can provide comfort and stability in a threatening and unnatural environment. Family Support. Providing support and reassurance to parents is among the major nursing responsibilities. The seriousness and emergency nature of AKI are stressful to parents, and most feel some degree of guilt regarding the child's condition, especially when the illness is the result of ingestion of a toxic substance, dehydration, or a genetic disease. They need reassurance and an empathetic listener. They also need to be kept informed of the child's progress and provided explanations regarding the therapeutic regimen. The equipment and the child's behavior are sometimes frightening and anxiety provoking. Nurses can do much to help parents comprehend and deal with the stresses of the situation.
ChatGPT said:
ChatGPT
Acute Kidney Injury (AKI) Overview
Definition: Acute Kidney Injury (AKI) occurs when the kidneys suddenly lose their ability to regulate urine volume and composition in response to food and fluid intake. Key features include oligoanuria (reduced urine output), azotemia (elevated nitrogenous waste in the blood), acidosis, and electrolyte imbalances. While not common in children, early recognition and treatment are crucial for favorable outcomes.
Etiology
AKI can arise from various clinical conditions, often classified into three categories:
Prerenal Causes:
Description: Related to reduced renal perfusion in a structurally normal kidney.
Common Causes:
Dehydration (from diarrhea or vomiting)
Surgical shock
Trauma (including burns)
Mechanism: Decreased renal perfusion leads to reduced glomerular filtration rate (GFR) and compensatory hormonal responses, potentially causing cortical or tubular necrosis if prolonged.
Intrinsic Renal Causes:
Description: Involves direct damage to the kidney tissues (glomeruli, tubules, or vasculature).
Common Causes:
Glomerular diseases (e.g., glomerulonephritis)
Tubular necrosis (ischemia or nephrotoxins)
Mechanism: Varies depending on the type and extent of damage, leading to varying degrees of renal dysfunction.
Postrenal Causes:
Description: Resulting from obstruction in the urinary tract.
Common Causes: Rare in children but may occur within the first year of life.
Mechanism: Relief of obstruction may restore function; duration of obstruction affects recovery.
Pathophysiology
Glomerular Filtration Rate (GFR): Severely reduced, leading to elevated Blood Urea Nitrogen (BUN) and altered sodium reabsorption.
Hormonal Responses: Increased renin secretion causes afferent arteriolar vasoconstriction, further reducing GFR.
Necrosis Types:
Cortical Necrosis: Severe ischemia or infections; most common in neonates.
Tubular Necrosis: Ischemia and nephrotoxic injuries, resulting in tubular epithelial cell death and possible incomplete recovery.
Clinical Course
Phases of AKI:
Oliguric Phase: Low urine output (<1 ml/kg/hr) with possible anuria.
Diuretic Phase: Abrupt increase in urine output, potentially leading to electrolyte imbalances.
Duration of the oliguric phase can vary significantly based on underlying causes.
Clinical Manifestations
Key Symptoms:
Oliguria or anuria
Edema
Drowsiness
Hypertension
Cardiac arrhythmias due to hyperkalemia
Neurologic symptoms (seizures, altered mental status)
Diagnostic Evaluation
Key Laboratory Findings:
Azotemia: Elevated BUN and creatinine due to decreased excretion.
Electrolyte Imbalances: Hyperkalemia, hyponatremia, metabolic acidosis.
Anemia: Due to chronic illness or renal dysfunction.
Therapeutic Management
Prevention: Recognize at-risk situations and implement fluid therapy and nephrotoxin precautions.
Fluid and Electrolyte Management:
Restore volume for prerenal causes.
Monitor and restrict electrolytes based on levels and patient needs.
Management of Hyperkalemia:
Monitor serum potassium levels and ECG changes.
Temporary interventions: Calcium gluconate, sodium bicarbonate, insulin and glucose.
Definitive treatment: Sodium polystyrene sulfonate (Kayexalate) or dialysis.
Blood Pressure Control:
Monitor blood pressure every 4–6 hours.
Use antihypertensive medications (e.g., labetalol, sodium nitroprusside) as needed.
Nutritional Support:
Provide high-calorie, low-protein, low-potassium diets.
IV nutritional support if oral intake is insufficient.
Monitoring and Supportive Care:
Intensive monitoring of fluid balance, vital signs, and laboratory values.
Family support and education regarding the child's condition.
Prognosis
Outcomes: Dependent on the cause, severity, and timeliness of management. Mortality rates are generally less than 20%, with favorable outcomes expected in cases of dehydration or nephrotoxins, while HUS or rapidly progressive nephritis may lead to long-term complications.
Nursing Care Management
Focus on monitoring fluid and electrolyte balance, preventing complications, and providing family support.
Prepare for potential dialysis needs and ensure comprehensive care coordination in intensive settings.
Offer empathetic support to both child and family, addressing emotional and informational needs during treatment.
4o mini
You said:
Laboratory Findings Associated With Acute Renal Failure Clinical Problem Mechanism Clinical Considerations Azotemia Ongoing protein catabolism Lower rate of production in neonates and persons with depleted protein stores Elevated blood urea nitrogen levels Significantly decreased excretion Increased in situations involving large amounts of necrotic tissue or extravasated blood Elevated plasma creatinine levels Continued production Significantly decreased excretion Production less affected by other factors More sensitive measure of intensity of azotemia Low in neonate because of small muscle mass relative to size Metabolic acidosis Continued endogenous acid production Significantly decreased excretion Depletion of extracellular and intracellular fluid buffers Compensatory hyperventilation Opisthonos Major threat to life Hyponatremia Dilution of extracellular fluid Decreased excretion of water May develop cerebral signs Hyperkalemia Ongoing protein catabolism Decreased excretion compounded by metabolic acidosis Most important electrolyte to be considered in acute renal failure May contribute to cardiac arrhythmia With electrocardiogram changes, major threat to life Loss may be from gastrointestinal tract Hypocalcemia Associated with metabolic acidosis and hyperphosphatemia During alkali therapy, may cause tetany
ChatGPT said:
ChatGPT
Laboratory Findings Associated With Acute Renal Failure
Clinical ProblemMechanismClinical Considerations | ||
Azotemia | Ongoing protein catabolism | Lower rate of production in neonates and individuals with depleted protein stores. |
Elevated blood urea nitrogen levels | Significantly decreased excretion; increased in situations involving large amounts of necrotic tissue or extravasated blood. | |
Elevated plasma creatinine levels | Continued production; significantly decreased excretion; production less affected by other factors. More sensitive measure of intensity of azotemia; levels are low in neonates due to small muscle mass relative to size. | |
Metabolic acidosis | Continued endogenous acid production | Significantly decreased excretion; depletion of extracellular and intracellular fluid buffers; compensatory hyperventilation; opisthonos; major threat to life. |
Hyponatremia | Dilution of extracellular fluid | Decreased excretion of water; may develop cerebral signs. |
Hyperkalemia | Ongoing protein catabolism | Decreased excretion compounded by metabolic acidosis; most important electrolyte to consider in acute renal failure; may contribute to cardiac arrhythmia; with electrocardiogram changes, poses a major threat to life. Loss may occur from the gastrointestinal tract. |
Hypocalcemia | Associated with metabolic acidosis and hyperphosphatemia | During alkali therapy, may cause tetany. |
This table summarizes the key laboratory findings that indicate the presence of acute renal failure, including the underlying mechanisms and clinical considerations for each abnormality. Monitoring these findings is crucial for the management and treatment of patients with acute kidney injury.
4o mini
You said:
Discuss laboratory findings associated with acute renal failure.
ChatGPT said:
ChatGPT
Acute renal failure (ARF), also known as acute kidney injury (AKI), is characterized by a sudden decline in kidney function, which leads to the accumulation of waste products in the blood and an imbalance of electrolytes and fluids. Various laboratory findings are associated with ARF, reflecting the underlying pathophysiology and guiding management. Here’s a discussion of the key laboratory findings:
1. Azotemia
Definition: Azotemia refers to an increase in blood urea nitrogen (BUN) and creatinine levels due to the accumulation of nitrogenous waste products in the bloodstream.
Mechanism: In ARF, there is a combination of ongoing protein catabolism and significantly decreased renal excretion.
Clinical Considerations:
BUN levels can rise significantly in conditions with extensive tissue necrosis (e.g., rhabdomyolysis) or significant blood loss.
Creatinine levels are a more sensitive marker for kidney function, as their production is less influenced by diet and muscle mass. In neonates, creatinine levels may be low due to a smaller muscle mass.
2. Electrolyte Abnormalities
Hyponatremia:
Mechanism: Often occurs due to the dilution of extracellular fluid or impaired renal excretion of water.
Clinical Considerations: Can lead to neurological symptoms, including seizures or confusion, if severe.
Hyperkalemia:
Mechanism: Results from ongoing protein catabolism and decreased renal excretion, particularly in the context of metabolic acidosis.
Clinical Considerations: Hyperkalemia is particularly dangerous as it can cause life-threatening cardiac arrhythmias. Electrocardiogram (ECG) changes (e.g., peaked T-waves) are critical indicators.
Hypocalcemia:
Mechanism: Often associated with metabolic acidosis and hyperphosphatemia, where phosphate levels rise and bind calcium, leading to decreased serum calcium.
Clinical Considerations: Hypocalcemia can lead to tetany, especially during alkalizing treatment for acidosis.
3. Metabolic Acidosis
Mechanism: ARF leads to decreased excretion of acids and ongoing endogenous acid production, which results in a lower blood pH.
Clinical Considerations: Patients may exhibit compensatory mechanisms, such as hyperventilation. Severe acidosis can result in muscle spasms (opisthonos) and represents a significant threat to life.
4. Other Laboratory Findings
Urinalysis:
Changes in urine output (oliguria or anuria) are common. Urinalysis may show casts, protein, or blood depending on the underlying cause of renal failure.
Imaging Studies:
While not laboratory tests per se, imaging studies such as ultrasound can help assess for obstructions or abnormalities in renal structure.
Summary
The laboratory findings associated with acute renal failure are critical for diagnosing and managing the condition. Elevated levels of BUN and creatinine indicate decreased kidney function, while electrolyte imbalances such as hyperkalemia and hyponatremia highlight the acute nature of renal impairment and its systemic effects. Monitoring these parameters allows healthcare providers to guide treatment effectively and mitigate potential complications.
4o mini
You said:
Compare and contrast the pathophysiology, clinical manifestations, and nursing management of children with acute renal failure and with chronic renal failure (CRF). Chronic Kidney Disease The kidneys are able to maintain the chemical composition of fluids within normal limits until more than 50% of functional renal capacity is destroyed by disease or injury. Chronic kidney disease (CKD) occurs when the diseased kidneys can no longer maintain the normal chemical structure of body fluids under normal conditions. Progressive deterioration over months or years produces a variety of clinical and biochemical disturbances that conclude in the clinical syndrome known as uremia. The final stage of CKD, ESRD, is irreversible. Treatment with dialysis or transplantation is required when the glomerular filtration rate decreases below 10% to 15% of normal. The pattern of renal dysfunction is remarkably uniform no matter what disease process initiates the advanced disease. Etiology A variety of diseases and disorders can result in CKD. The most common causes of CKD before age 5 years are congenital renal and urinary tract malformations (particularly renal hypoplasia and dysplasia and obstructive uropathy) and VUR. Glomerular and hereditary renal diseases predominate in children 5 to 15 years of age. The glomerular diseases that most commonly lead to CKD are chronic pyelonephritis, CGN, and glomerulonephropathy associated with systemic diseases such as anaphylactoid purpura and lupus erythematosus. Hereditary nephritis, congenital nephrotic syndrome, Alport syndrome, polycystic kidney, and several other hereditary disorders result in renal failure in childhood. Renal vascular disorders such as HUS, vascular thrombosis, or cortical necrosis are less common causes. Pathophysiology Early in the course of progressive nephron destruction, the child remains asymptomatic with only minimum biochemical abnormalities. Unless its presence is detected in the process of routine assessment, signs and symptoms that indicate advanced renal damage often emerge only late in the course of the disease. Midway in the disease process, as increasing numbers of nephrons are totally destroyed, and most others are damaged to varying degrees; the few that remain intact are hypertrophied but functional. These few normal nephrons are able to make sufficient adjustments to stresses to maintain reasonable degrees of fluid and electrolyte balance. Definitive biochemical examination at this time reveals restricted tolerance to excesses or restrictions. As the disease progresses to the end stage because of severe reduction in the number of functioning nephrons, the kidneys are no longer able to maintain fluid and electrolyte balance, and the features of uremia appear. The following sections briefly summarize the pathophysiology of specific biochemical abnormalities. Retention of Waste Products. Serum creatinine and BUN levels are utilized to evaluate renal function. Creatinine is a waste product of muscle catabolism. Because muscle mass is relatively stable, creatinine production is also stable. Most creatinine is filtered out by the kidneys and expelled in the urine. BUN (a by-product of protein breakdown) levels also increase as kidney function declines. BUN is a less precise marker than creatinine as is not produced at a stable rate and can be influenced by protein intake, hydration status and other factors (Lopez-Giacoman & Madero, 2015). Water and Sodium Retention. The damaged kidneys are able to maintain sodium and water balance under normal circumstances, although the few remaining functional nephrons are required to increase their rate of filtration and reabsorption in proportion to their numbers. The limitations of this capacity become apparent under stress. The nature of abnormalities in adjustment depends on the underlying renal disease. Infants and small children with kidney dysplasia or urinary obstructive disease tend to excrete large volumes of dilute urine low in sodium content. Children with glomerular disease tend to retain both sodium and water as a result of a greater reduction of glomerular filtration than of tubular reabsorption. Children with defective sodium reabsorption from tubular disease tend to lose sodium, with a corresponding osmotic water loss. Consequently, sodium excesses may cause edema and hypertension, whereas sodium deprivation can result in hypovolemia and circulatory failure. Only in ESRD is markedly reduced glomerular filtration inadequate to handle normal amounts of sodium and water. Retention of these substances leads to edema and vascular congestion. Hyperkalemia. Dangerous hyperkalemia is uncommon in CKD until the end stage. However, the kidneys are unable to adjust readily to increased ingestion of potassium, and they require a longer period to rid the body of this excess. Acidosis. A sustained metabolic acidosis is characteristic of CKD; it results from the damaged kidney's inability to excrete a normal load of metabolic acids generated by normal metabolic processes. There is reduced capacity of the distal tubules to produce ammonia and impaired reabsorption of bicarbonate. Despite continuous hydrogen ion retention and bicarbonate loss, the plasma pH is maintained at a level compatible with life by other buffering mechanisms, particularly the bone salt (see the following sections). Calcium and Phosphorus Disturbances. Calcium and phosphorus homeostasis are affected by CKD. Profound and complex disturbances in the metabolism of these substances result in significant bone demineralization and impaired growth. This appears to be related to several factors (Box 24.8). These complex disturbances in calcium, phosphorus, and bone metabolism produce growth arrest or delay; bone pain; and deformities known as renal osteodystrophy, sometimes called renal rickets, because the disorganization of bone growth and demineralization are similar to that caused by vitamin D–resistant rickets. Box 24.8 Factors Related to Bone Demineralization in Chronic Renal Failure • In a state of acidosis there is dissolution of the alkaline salts of bone, which serve as buffers, and the release of phosphorus and calcium into the bloodstream. • Reduced glomerular filtration and excretion of inorganic phosphate lead to an elevation of plasma phosphate with a concomitant decrease in serum calcium. • Decreased serum calcium concentration stimulates the secretion of parathyroid hormone, which results in reabsorption of calcium from bones. Under normal circumstances parathyroid hormone inhibits the tubular reabsorption of phosphates. • Diseased kidneys are unable to complete the synthesis of vitamin D to its most active form, 1,25-dihydroxycholecalciferol, which is necessary for the absorption of calcium from the gastrointestinal tract and deposition of calcium in bone. This acquired resistance to vitamin D decreases calcium absorption, permits retention of phosphorus, and contributes to secondary hyperparathyroidism. Anemia. A consistent feature of CKD is anemia, which appears to result from several factors (Box 24.9). Box 24.9 Causes of Anemia in Chronic Renal Failure • Shortened life span of red blood cells caused by some extracorpuscular factor associated with the uremic state • Impaired red blood cell production resulting from decreased production of erythropoietin • Blood loss related to increased tendency to bleed, associated with a prolonged bleeding time, probably related to impaired platelet function and laboratory blood samples • Hyperparathyroidism • Hypersplenism, which may be related to silicone deposition (from dialysis blood lines) and granuloma formation in the spleen • Diseases related to hemolytic anemia, such as systemic lupus erythematosus and sickle cell disease Growth Disturbance. One of the most striking effects of CKD in childhood, and one that can have profound psychologic and social consequences for the developing child, is delayed growth. The cause is poorly understood but may be related to nutritional and biochemical factors (Box 24.10). Box 24.10 Probable Causes of Growth Failure in Chronic Renal Failure • Renal osteodystrophy • Poor nutrition associated with dietary restrictions (especially protein) and loss of appetite • Biochemical abnormalities associated with renal failure, such as sustained acidosis or renal sodium wasting • Hypertension • Corticosteroid treatment • Tissue resistance to growth hormone • Trace mineral and vitamin deficiencies Sexual maturation may be delayed or may not occur in children with CKD, and secondary amenorrhea commonly develops in girls past puberty. CKD can also cause sexual dysfunction by creating imbalances in gonadal hormone levels. Decreased testosterone levels impair spermatogenesis in males; decreased estrogen, luteinizing hormone, and progesterone cause anovulation and menstrual irregularities (usually amenorrhea) in females. Autonomic neuropathy and anemia are also factors that can alter sexual function. Other Disturbances. Children with CKD are more susceptible to infection, especially pneumonia, UTI, and septicemia, although the reason for this is not entirely clear. Hyperventilation, a manifestation of the respiratory compensatory mechanism for metabolic acidosis, and pulmonary edema may contribute to upper respiratory tract infection. These children become extraordinarily sensitive to changes in vascular volume that may cause, in addition to pulmonary overload, cerebral symptoms and circulatory manifestations such as hypertension and cardiac failure. Numerous neurologic manifestations appear with advanced renal failure, although no specific toxin or biochemical defect has been identified. However, disturbances in enzyme function, disturbances in water and electrolyte balance, altered calcium ion concentration, hypertension, and accumulation of various “uremic toxins” have been implicated. Clinical Manifestations The first symptom of CKD may be loss of normal energy and increased fatigue on exertion. For example, the child may prefer quiet, passive activities rather than participation in more active games and outdoor play. The child is usually somewhat pale, but the change is often so subtle that it may not be evident to parents or others. Blood pressure is sometimes elevated. Growth is affected early in the development of CKD, and falling behind on the growth chart is often the first measurable sign. Other manifestations may appear as the disease progresses. The child does not eat as well (especially breakfast), shows less interest in normal activities such as schoolwork or play, and has a decreased or increased urinary output and a compensatory intake of fluid. For example, a child who has achieved bladder control may wet the bed at night. Pallor becomes more evident as the skin develops a characteristic sallow, muddy appearance as a result of anemia and deposition of urochrome pigment in the skin. The child may complain of headache, muscle cramps, and nausea. Other signs and symptoms include weight loss, facial puffiness, malaise, bone or joint pain, growth retardation, dryness or itching of the skin, bruised skin, and sometimes sensory or motor loss. Amenorrhea is common in adolescent girls. Therapy is generally initiated before the appearance of the uremic symptoms, although on some occasions the symptoms may be observed. Manifestations of untreated uremia reflect the progressive nature of the homeostatic disturbances and general toxicity. Gastrointestinal symptoms include loss of appetite, nausea, and vomiting. Bleeding tendencies are apparent in bruises, bloody diarrheal stools, stomatitis, and bleeding from the lips and mouth. Intractable itching occurs, probably related to a number of factors, including dry skin and hyperparathyroidism (Wojtowicz-Prus, Kiliś-Pstrusińska, Reich, et al., 2016). Deposits of urea crystals may appear on the skin as uremic frost but are seldom seen because of the availability of dialysis and transplantation. There may be an unpleasant uremic odor to the breath. Respirations become deeper as a result of metabolic acidosis, and circulatory overload is manifested by hypertension, congestive heart failure, and pulmonary edema. Progressive confusion, dulling of the sensorium, and ultimately coma are signs of neurologic involvement. Other signs may include tremors, muscular twitching, and seizures. Diagnostic Evaluation The diagnosis of CKD is usually suspected on the basis of any of a number of clinical manifestations, a history of prior renal disease, or biochemical findings. The onset is usually gradual, and the initial signs and symptoms are vague and nonspecific. Laboratory and other diagnostic tools and tests are of value in assessing the extent of renal damage, biochemical disturbances, and related physical dysfunction. Often, they can help establish the nature of the underlying disease and differentiate between other disease processes and the pathologic consequences of renal dysfunction. Therapeutic Management Classification of CKD as stage 1 (GFR ≥90) through stage 5 (GFR <15 or dialysis) helps with evaluation and management decisions through the use of medical protocols such as the National Kidney Foundation Kidney Disease Outcomes Quality Initiative evidence-based clinical practice guidelines (http://www.kidney.org/professionals/KDOQI). The goals of management are to maximize effective renal function, maintain body fluid and electrolyte balance within acceptable limits, treat systemic complications, and promote as active and normal a life as possible for the child for as long as possible. This becomes increasingly difficult as the disease progresses toward end stage. Therapeutic measures designed to relieve one manifestation may negatively affect another. For example, antihypertensive agents may further impair renal function. Activity. Allow children unrestricted activity and to set their own limits regarding rest and extent of exertion. Encourage them to attend school. If the effort is too great, home tutoring can be arranged. Diet. Regulation of diet has been seen as the most effective means, short of dialysis, for reducing the quantity of materials that require renal excretion. The goal of the diet in renal failure is to provide sufficient calories and protein for growth while minimizing the excretory demands made on the kidney, to limit metabolic bone disease (osteodystrophy), and to minimize fluid and electrolyte disturbances. Dietary protein intake is limited to the recommended dietary allowance (RDA) for the child's age. Restriction of protein intake below the RDA is believed to negatively affect growth and neurodevelopment. Dietary phosphorus may need to be restricted. Remember that any attempt to restrict dietary intake in children potentially restricts caloric intake and can limit growth. Protein in the diet should include foods of high biologic value. When given with meals, substances that bind phosphorus in the intestines prevent its absorption and allow a more liberal intake of phosphorus-containing protein. Sodium and water are not usually limited unless there is evidence of edema or hypertension. Potassium is not restricted as long as creatinine clearance remains at acceptable limits (30 to 35 ml/min). However, restrictions are instituted for patients with oliguria or anuria. Restrictions of any or all of these minerals may be imposed in later stages or at any time in which factors cause abnormal serum concentrations. Because of modified dietary intake, altered metabolism, and poor appetite, some dietary supplementation is usually needed. Because fat-soluble vitamins can accumulate in patients with CKD, vitamins A, E, and K are not supplemented beyond normal dietary intake. Active and/or inactive forms of vitamin D are prescribed, and water-soluble vitamin supplementation may be required if the diet is inadequate. Other dietary needs are discussed in relation to osteodystrophy and anemia. Dietary management of the child with renal failure is a difficult and complex problem that necessitates collaboration with a registered dietitian who is knowledgeable about pediatric nutrition and the impact of renal failure. Osteodystrophy. Measures directed at prevention or correction of the calcium/phosphorus imbalance are reduction of dietary phosphorus, administration of a phosphorus-binding agent, provision of supplemental calcium, control of acidosis, and administration of an active and/or inactive form of vitamin D. The reduction of protein and milk intake can control dietary phosphorus. Oral administration of phosphorus-binding agents, which combine with the phosphorus to decrease gastrointestinal absorption and thus the serum levels of phosphate, can further reduce phosphorus levels. Calcium carbonate preparations can be used as phosphorus binders. These medications act as phosphate binders, calcium supplements, and alkalizing agents. Calcium carbonate preparations can be given with meals to bind phosphorus if the child is hyperphosphatemic or mildly hypocalcemic. If given 1 to 2 hours after meals, they act as calcium supplements for children with stable phosphorus but low calcium levels. Calcium acetate can also be used. Newer, iron-based binders can be used when there is concern for hypercalcemia (Stormont, McCoy, Bashir, et al., 2016). Aluminum hydroxide gels are effective phosphorus binders but have been shown to cause aluminum loading when used in children with renal failure. Aluminum intoxication leads to altered sensorium, an inability to talk, ataxia, seizures, and severe bone disease. Secondary hyperparathyroidism (evidenced by an elevated intact PTH level), in a child with normal phosphate, calcium and 25OH vitamin D levels requires treatment with an active form of vitamin D. Oral medications such as calcitriol (Rocaltrol) increase absorption of calcium through the gastrointestinal tract. The serum calcium level is monitored frequently during periods when the drugs are being initiated or changed to detect hypercalcemia. Intact parathyroid hormone levels are measured every 2 to 3 months with target levels based on the stage of CKD. Osseous deformities that result from renal osteodystrophy, especially those related to ambulation, are troublesome and require correction if they occur. Careful attention to the management of osteodystrophy and bone growth can prevent deformities in some children. Acidosis. Pharmacologic treatment of acidosis is initiated early in children who have chronic renal insufficiency. In addition to reducing the formation of metabolic acids by avoiding excessive dietary protein intake, alkalizing agents such as sodium bicarbonate or a combination of sodium and potassium citrate (Bicitra, Polycitra, or Shohl solution) alleviate acidosis. Correction of acidosis is best attempted after calcium levels are elevated because rapid correction may precipitate tetany in a hypocalcemic child. Anemia. Because the anemia associated with renal failure is related to decreased production of erythropoietin, it usually cannot be successfully managed with hematinic agents. Provide sufficient sources of folic acid and iron in the diet, although this is difficult when protein sources are restricted. Inadequate intake and iron losses that may occur are managed by supplemental iron, usually ferrous sulfate. Providing adequate sources of ascorbic acid at the same time that iron-rich foods or supplements are given enhances the absorption. The medication recombinant human erythropoietin (r-HuEPO) corrects anemia (improving energy level and general well-being) and eliminates the need for frequent blood transfusions in patients with CKD. To support the formation of new red blood cells before r-HuEPO therapy, iron stores must be adequate. Iron supplements are required in conjunction with r-HuEPO.
ChatGPT said:
ChatGPT
Comparison of Acute Renal Failure (ARF) and Chronic Renal Failure (CRF) in Children
Acute Renal Failure (ARF) and Chronic Renal Failure (CRF) are distinct conditions with different pathophysiological mechanisms, clinical manifestations, and nursing management strategies. Here's a detailed comparison and contrast between the two.
Pathophysiology
Acute Renal Failure (ARF):
Onset: Rapid, usually occurring over hours to days.
Mechanism: Often caused by factors such as acute tubular necrosis (due to ischemia or toxins), prerenal causes (e.g., dehydration), or postrenal obstruction (e.g., urinary tract blockage).
Nephron Function: In ARF, there is a sudden decrease in kidney function leading to the accumulation of waste products. Nephrons may still be capable of recovery if the underlying cause is identified and treated early.
Retention of Waste Products: Increased blood urea nitrogen (BUN) and creatinine levels reflect impaired excretion.
Chronic Renal Failure (CRF):
Onset: Gradual, often developing over months to years.
Mechanism: Caused by ongoing renal injury (e.g., congenital malformations, glomerulonephritis, systemic diseases) leading to progressive nephron loss.
Nephron Function: More than 50% of nephron function is lost before clinical symptoms appear. The remaining nephrons compensate initially but ultimately fail.
Retention of Waste Products: Leads to uremia, characterized by increased BUN and creatinine, as well as electrolyte imbalances, including acidosis and disturbances in calcium and phosphate metabolism.
Clinical Manifestations
Acute Renal Failure (ARF):
Symptoms: Sudden onset of oliguria or anuria, edema, hypertension, nausea, vomiting, and altered mental status.
Electrolyte Imbalances: May show hyperkalemia, hyponatremia, and metabolic acidosis.
Overall Health: Symptoms are often more acute and severe due to rapid accumulation of toxins.
Chronic Renal Failure (CRF):
Symptoms: Gradual development of fatigue, pallor, growth retardation, poor appetite, bone pain, and neurological manifestations (e.g., confusion, seizures).
Electrolyte Imbalances: Characterized by hyperphosphatemia, hypocalcemia, and metabolic acidosis, leading to complications such as renal osteodystrophy and anemia.
Overall Health: Symptoms can be subtle and may not be noticeable until significant renal damage has occurred, with systemic effects developing gradually.
Nursing Management
Acute Renal Failure (ARF):
Monitoring: Close monitoring of vital signs, fluid intake and output, and laboratory values (BUN, creatinine, electrolytes).
Intervention: Immediate identification and treatment of underlying causes (e.g., fluid resuscitation, removal of nephrotoxins, management of obstruction).
Supportive Care: May require temporary dialysis to manage severe electrolyte imbalances or fluid overload.
Education: Educate families about the signs of renal failure and the importance of early intervention.
Chronic Renal Failure (CRF):
Monitoring: Regular monitoring of growth parameters, blood pressure, laboratory values (including hemoglobin, calcium, phosphorus, and vitamin D levels).
Intervention: Dietary management to limit protein, phosphorus, and sodium intake; supplementation of vitamins and minerals as needed; and medications to manage anemia (e.g., erythropoietin) and bone metabolism (e.g., vitamin D, phosphate binders).
Long-term Management: Prepare for eventual dialysis or kidney transplantation as renal function declines.
Education: Teach families about the importance of adhering to dietary restrictions, recognizing signs of complications, and understanding the disease process.
Summary
In summary, ARF is characterized by a rapid onset of kidney dysfunction with the potential for recovery, while CRF involves a gradual decline in kidney function with progressive and irreversible damage. The nursing management of ARF focuses on acute interventions and stabilization, whereas CRF requires long-term care strategies aimed at managing chronic complications and preserving quality of life. Both conditions require vigilant monitoring and education for families to support optimal health outcomes.
4o mini
You said:
Chronic Kidney Disease The kidneys are able to maintain the chemical composition of fluids within normal limits until more than 50% of functional renal capacity is destroyed by disease or injury. Chronic kidney disease (CKD) occurs when the diseased kidneys can no longer maintain the normal chemical structure of body fluids under normal conditions. Progressive deterioration over months or years produces a variety of clinical and biochemical disturbances that conclude in the clinical syndrome known as uremia. The final stage of CKD, ESRD, is irreversible. Treatment with dialysis or transplantation is required when the glomerular filtration rate decreases below 10% to 15% of normal. The pattern of renal dysfunction is remarkably uniform no matter what disease process initiates the advanced disease. Etiology A variety of diseases and disorders can result in CKD. The most common causes of CKD before age 5 years are congenital renal and urinary tract malformations (particularly renal hypoplasia and dysplasia and obstructive uropathy) and VUR. Glomerular and hereditary renal diseases predominate in children 5 to 15 years of age. The glomerular diseases that most commonly lead to CKD are chronic pyelonephritis, CGN, and glomerulonephropathy associated with systemic diseases such as anaphylactoid purpura and lupus erythematosus. Hereditary nephritis, congenital nephrotic syndrome, Alport syndrome, polycystic kidney, and several other hereditary disorders result in renal failure in childhood. Renal vascular disorders such as HUS, vascular thrombosis, or cortical necrosis are less common causes. Pathophysiology Early in the course of progressive nephron destruction, the child remains asymptomatic with only minimum biochemical abnormalities. Unless its presence is detected in the process of routine assessment, signs and symptoms that indicate advanced renal damage often emerge only late in the course of the disease. Midway in the disease process, as increasing numbers of nephrons are totally destroyed, and most others are damaged to varying degrees; the few that remain intact are hypertrophied but functional. These few normal nephrons are able to make sufficient adjustments to stresses to maintain reasonable degrees of fluid and electrolyte balance. Definitive biochemical examination at this time reveals restricted tolerance to excesses or restrictions. As the disease progresses to the end stage because of severe reduction in the number of functioning nephrons, the kidneys are no longer able to maintain fluid and electrolyte balance, and the features of uremia appear. The following sections briefly summarize the pathophysiology of specific biochemical abnormalities. Retention of Waste Products. Serum creatinine and BUN levels are utilized to evaluate renal function. Creatinine is a waste product of muscle catabolism. Because muscle mass is relatively stable, creatinine production is also stable. Most creatinine is filtered out by the kidneys and expelled in the urine. BUN (a by-product of protein breakdown) levels also increase as kidney function declines. BUN is a less precise marker than creatinine as is not produced at a stable rate and can be influenced by protein intake, hydration status and other factors (Lopez-Giacoman & Madero, 2015). Water and Sodium Retention. The damaged kidneys are able to maintain sodium and water balance under normal circumstances, although the few remaining functional nephrons are required to increase their rate of filtration and reabsorption in proportion to their numbers. The limitations of this capacity become apparent under stress. The nature of abnormalities in adjustment depends on the underlying renal disease. Infants and small children with kidney dysplasia or urinary obstructive disease tend to excrete large volumes of dilute urine low in sodium content. Children with glomerular disease tend to retain both sodium and water as a result of a greater reduction of glomerular filtration than of tubular reabsorption. Children with defective sodium reabsorption from tubular disease tend to lose sodium, with a corresponding osmotic water loss. Consequently, sodium excesses may cause edema and hypertension, whereas sodium deprivation can result in hypovolemia and circulatory failure. Only in ESRD is markedly reduced glomerular filtration inadequate to handle normal amounts of sodium and water. Retention of these substances leads to edema and vascular congestion. Hyperkalemia. Dangerous hyperkalemia is uncommon in CKD until the end stage. However, the kidneys are unable to adjust readily to increased ingestion of potassium, and they require a longer period to rid the body of this excess. Acidosis. A sustained metabolic acidosis is characteristic of CKD; it results from the damaged kidney's inability to excrete a normal load of metabolic acids generated by normal metabolic processes. There is reduced capacity of the distal tubules to produce ammonia and impaired reabsorption of bicarbonate. Despite continuous hydrogen ion retention and bicarbonate loss, the plasma pH is maintained at a level compatible with life by other buffering mechanisms, particularly the bone salt (see the following sections). Calcium and Phosphorus Disturbances. Calcium and phosphorus homeostasis are affected by CKD. Profound and complex disturbances in the metabolism of these substances result in significant bone demineralization and impaired growth. This appears to be related to several factors (Box 24.8). These complex disturbances in calcium, phosphorus, and bone metabolism produce growth arrest or delay; bone pain; and deformities known as renal osteodystrophy, sometimes called renal rickets, because the disorganization of bone growth and demineralization are similar to that caused by vitamin D–resistant rickets. Box 24.8 Factors Related to Bone Demineralization in Chronic Renal Failure • In a state of acidosis there is dissolution of the alkaline salts of bone, which serve as buffers, and the release of phosphorus and calcium into the bloodstream. • Reduced glomerular filtration and excretion of inorganic phosphate lead to an elevation of plasma phosphate with a concomitant decrease in serum calcium. • Decreased serum calcium concentration stimulates the secretion of parathyroid hormone, which results in reabsorption of calcium from bones. Under normal circumstances parathyroid hormone inhibits the tubular reabsorption of phosphates. • Diseased kidneys are unable to complete the synthesis of vitamin D to its most active form, 1,25-dihydroxycholecalciferol, which is necessary for the absorption of calcium from the gastrointestinal tract and deposition of calcium in bone. This acquired resistance to vitamin D decreases calcium absorption, permits retention of phosphorus, and contributes to secondary hyperparathyroidism. Anemia. A consistent feature of CKD is anemia, which appears to result from several factors (Box 24.9). Box 24.9 Causes of Anemia in Chronic Renal Failure • Shortened life span of red blood cells caused by some extracorpuscular factor associated with the uremic state • Impaired red blood cell production resulting from decreased production of erythropoietin • Blood loss related to increased tendency to bleed, associated with a prolonged bleeding time, probably related to impaired platelet function and laboratory blood samples • Hyperparathyroidism • Hypersplenism, which may be related to silicone deposition (from dialysis blood lines) and granuloma formation in the spleen • Diseases related to hemolytic anemia, such as systemic lupus erythematosus and sickle cell disease Growth Disturbance. One of the most striking effects of CKD in childhood, and one that can have profound psychologic and social consequences for the developing child, is delayed growth. The cause is poorly understood but may be related to nutritional and biochemical factors (Box 24.10). Box 24.10 Probable Causes of Growth Failure in Chronic Renal Failure • Renal osteodystrophy • Poor nutrition associated with dietary restrictions (especially protein) and loss of appetite • Biochemical abnormalities associated with renal failure, such as sustained acidosis or renal sodium wasting • Hypertension • Corticosteroid treatment • Tissue resistance to growth hormone • Trace mineral and vitamin deficiencies Sexual maturation may be delayed or may not occur in children with CKD, and secondary amenorrhea commonly develops in girls past puberty. CKD can also cause sexual dysfunction by creating imbalances in gonadal hormone levels. Decreased testosterone levels impair spermatogenesis in males; decreased estrogen, luteinizing hormone, and progesterone cause anovulation and menstrual irregularities (usually amenorrhea) in females. Autonomic neuropathy and anemia are also factors that can alter sexual function. Other Disturbances. Children with CKD are more susceptible to infection, especially pneumonia, UTI, and septicemia, although the reason for this is not entirely clear. Hyperventilation, a manifestation of the respiratory compensatory mechanism for metabolic acidosis, and pulmonary edema may contribute to upper respiratory tract infection. These children become extraordinarily sensitive to changes in vascular volume that may cause, in addition to pulmonary overload, cerebral symptoms and circulatory manifestations such as hypertension and cardiac failure. Numerous neurologic manifestations appear with advanced renal failure, although no specific toxin or biochemical defect has been identified. However, disturbances in enzyme function, disturbances in water and electrolyte balance, altered calcium ion concentration, hypertension, and accumulation of various “uremic toxins” have been implicated. Clinical Manifestations The first symptom of CKD may be loss of normal energy and increased fatigue on exertion. For example, the child may prefer quiet, passive activities rather than participation in more active games and outdoor play. The child is usually somewhat pale, but the change is often so subtle that it may not be evident to parents or others. Blood pressure is sometimes elevated. Growth is affected early in the development of CKD, and falling behind on the growth chart is often the first measurable sign. Other manifestations may appear as the disease progresses. The child does not eat as well (especially breakfast), shows less interest in normal activities such as schoolwork or play, and has a decreased or increased urinary output and a compensatory intake of fluid. For example, a child who has achieved bladder control may wet the bed at night. Pallor becomes more evident as the skin develops a characteristic sallow, muddy appearance as a result of anemia and deposition of urochrome pigment in the skin. The child may complain of headache, muscle cramps, and nausea. Other signs and symptoms include weight loss, facial puffiness, malaise, bone or joint pain, growth retardation, dryness or itching of the skin, bruised skin, and sometimes sensory or motor loss. Amenorrhea is common in adolescent girls. Therapy is generally initiated before the appearance of the uremic symptoms, although on some occasions the symptoms may be observed. Manifestations of untreated uremia reflect the progressive nature of the homeostatic disturbances and general toxicity. Gastrointestinal symptoms include loss of appetite, nausea, and vomiting. Bleeding tendencies are apparent in bruises, bloody diarrheal stools, stomatitis, and bleeding from the lips and mouth. Intractable itching occurs, probably related to a number of factors, including dry skin and hyperparathyroidism (Wojtowicz-Prus, Kiliś-Pstrusińska, Reich, et al., 2016). Deposits of urea crystals may appear on the skin as uremic frost but are seldom seen because of the availability of dialysis and transplantation. There may be an unpleasant uremic odor to the breath. Respirations become deeper as a result of metabolic acidosis, and circulatory overload is manifested by hypertension, congestive heart failure, and pulmonary edema. Progressive confusion, dulling of the sensorium, and ultimately coma are signs of neurologic involvement. Other signs may include tremors, muscular twitching, and seizures. Diagnostic Evaluation The diagnosis of CKD is usually suspected on the basis of any of a number of clinical manifestations, a history of prior renal disease, or biochemical findings. The onset is usually gradual, and the initial signs and symptoms are vague and nonspecific. Laboratory and other diagnostic tools and tests are of value in assessing the extent of renal damage, biochemical disturbances, and related physical dysfunction. Often, they can help establish the nature of the underlying disease and differentiate between other disease processes and the pathologic consequences of renal dysfunction. Therapeutic Management Classification of CKD as stage 1 (GFR ≥90) through stage 5 (GFR <15 or dialysis) helps with evaluation and management decisions through the use of medical protocols such as the National Kidney Foundation Kidney Disease Outcomes Quality Initiative evidence-based clinical practice guidelines (http://www.kidney.org/professionals/KDOQI). The goals of management are to maximize effective renal function, maintain body fluid and electrolyte balance within acceptable limits, treat systemic complications, and promote as active and normal a life as possible for the child for as long as possible. This becomes increasingly difficult as the disease progresses toward end stage. Therapeutic measures designed to relieve one manifestation may negatively affect another. For example, antihypertensive agents may further impair renal function. Activity. Allow children unrestricted activity and to set their own limits regarding rest and extent of exertion. Encourage them to attend school. If the effort is too great, home tutoring can be arranged. Diet. Regulation of diet has been seen as the most effective means, short of dialysis, for reducing the quantity of materials that require renal excretion. The goal of the diet in renal failure is to provide sufficient calories and protein for growth while minimizing the excretory demands made on the kidney, to limit metabolic bone disease (osteodystrophy), and to minimize fluid and electrolyte disturbances. Dietary protein intake is limited to the recommended dietary allowance (RDA) for the child's age. Restriction of protein intake below the RDA is believed to negatively affect growth and neurodevelopment. Dietary phosphorus may need to be restricted. Remember that any attempt to restrict dietary intake in children potentially restricts caloric intake and can limit growth. Protein in the diet should include foods of high biologic value. When given with meals, substances that bind phosphorus in the intestines prevent its absorption and allow a more liberal intake of phosphorus-containing protein. Sodium and water are not usually limited unless there is evidence of edema or hypertension. Potassium is not restricted as long as creatinine clearance remains at acceptable limits (30 to 35 ml/min). However, restrictions are instituted for patients with oliguria or anuria. Restrictions of any or all of these minerals may be imposed in later stages or at any time in which factors cause abnormal serum concentrations. Because of modified dietary intake, altered metabolism, and poor appetite, some dietary supplementation is usually needed. Because fat-soluble vitamins can accumulate in patients with CKD, vitamins A, E, and K are not supplemented beyond normal dietary intake. Active and/or inactive forms of vitamin D are prescribed, and water-soluble vitamin supplementation may be required if the diet is inadequate. Other dietary needs are discussed in relation to osteodystrophy and anemia. Dietary management of the child with renal failure is a difficult and complex problem that necessitates collaboration with a registered dietitian who is knowledgeable about pediatric nutrition and the impact of renal failure. Osteodystrophy. Measures directed at prevention or correction of the calcium/phosphorus imbalance are reduction of dietary phosphorus, administration of a phosphorus-binding agent, provision of supplemental calcium, control of acidosis, and administration of an active and/or inactive form of vitamin D. The reduction of protein and milk intake can control dietary phosphorus. Oral administration of phosphorus-binding agents, which combine with the phosphorus to decrease gastrointestinal absorption and thus the serum levels of phosphate, can further reduce phosphorus levels. Calcium carbonate preparations can be used as phosphorus binders. These medications act as phosphate binders, calcium supplements, and alkalizing agents. Calcium carbonate preparations can be given with meals to bind phosphorus if the child is hyperphosphatemic or mildly hypocalcemic. If given 1 to 2 hours after meals, they act as calcium supplements for children with stable phosphorus but low calcium levels. Calcium acetate can also be used. Newer, iron-based binders can be used when there is concern for hypercalcemia (Stormont, McCoy, Bashir, et al., 2016). Aluminum hydroxide gels are effective phosphorus binders but have been shown to cause aluminum loading when used in children with renal failure. Aluminum intoxication leads to altered sensorium, an inability to talk, ataxia, seizures, and severe bone disease. Secondary hyperparathyroidism (evidenced by an elevated intact PTH level), in a child with normal phosphate, calcium and 25OH vitamin D levels requires treatment with an active form of vitamin D. Oral medications such as calcitriol (Rocaltrol) increase absorption of calcium through the gastrointestinal tract. The serum calcium level is monitored frequently during periods when the drugs are being initiated or changed to detect hypercalcemia. Intact parathyroid hormone levels are measured every 2 to 3 months with target levels based on the stage of CKD. Osseous deformities that result from renal osteodystrophy, especially those related to ambulation, are troublesome and require correction if they occur. Careful attention to the management of osteodystrophy and bone growth can prevent deformities in some children. Acidosis. Pharmacologic treatment of acidosis is initiated early in children who have chronic renal insufficiency. In addition to reducing the formation of metabolic acids by avoiding excessive dietary protein intake, alkalizing agents such as sodium bicarbonate or a combination of sodium and potassium citrate (Bicitra, Polycitra, or Shohl solution) alleviate acidosis. Correction of acidosis is best attempted after calcium levels are elevated because rapid correction may precipitate tetany in a hypocalcemic child. Anemia. Because the anemia associated with renal failure is related to decreased production of erythropoietin, it usually cannot be successfully managed with hematinic agents. Provide sufficient sources of folic acid and iron in the diet, although this is difficult when protein sources are restricted. Inadequate intake and iron losses that may occur are managed by supplemental iron, usually ferrous sulfate. Providing adequate sources of ascorbic acid at the same time that iron-rich foods or supplements are given enhances the absorption. The medication recombinant human erythropoietin (r-HuEPO) corrects anemia (improving energy level and general well-being) and eliminates the need for frequent blood transfusions in patients with CKD. To support the formation of new red blood cells before r-HuEPO therapy, iron stores must be adequate. Iron supplements are required in conjunction with r-HuEPO. Hypertension. Hypertension of advanced renal disease may be managed initially by cautious use of a low-sodium diet, fluid restriction, and perhaps diuretics such as thiazides or furosemide. Strict restriction of sodium intake may be necessary in patients with oliguria. Severe hypertension may require the combination of a beta blocker and a vasodilator (propranolol and hydralazine). Other drugs that may be used include nifedipine, atenolol, minoxidil, prazosin, captopril, or labetalol, either singly or in combinations. Growth Retardation. One major consequence of CKD is growth retardation. Children with onset of renal failure earlier in life have more severe growth impairment than those diagnosed later (Rodig, McDermott, Schneider, et al., 2014). These children grow poorly both before and after initiation of dialysis. Assurance of adequate nutritional intake, correction of fluid and electrolyte imbalances, anemia, and metabolic acidosis should be undertaken. The use of recombinant human growth hormone has shown marked acceleration in growth velocity in children with growth retardation secondary to CKD (Rees, 2016). Miscellaneous Complications. Intercurrent infections are treated with appropriate antimicrobials. Most of these drugs are excreted through the kidneys; therefore the dosage is usually reduced in proportion to the decrease in renal function, and the interval between doses is extended in these children to avoid possible toxic effects from accumulation. Any drug eliminated through the kidneys is administered with caution. Serum levels of ototoxic or nephrotoxic drugs (e.g., aminoglycosides or vancomycin) are assessed regularly to ensure a safe, nontoxic level. Dental defects are common in children with chronic kidney disease; the earlier the onset of the disease, the more severe the dental manifestations. These defects include hypoplasia, hypomineralization, tooth discoloration, alteration in the size and shape of teeth, malocclusion (secondary to deficient skeletal growth), ulcerative stomatitis, occasional oral hematomas, and an increase in calcific deposits around the teeth. Regular dental care is especially important in these children. Other nondental complications are treated symptomatically—for example, chlorpromazine (Thorazine) or prochlorperazine (Compazine) is given for nausea, antiepileptics are given for seizures, and diphenhydramine (Benadryl) is given for pruritus. Once a child reaches CKD stage 5, death can occur unless waste products and toxins are removed from body fluids by dialysis or kidney transplantation. Since the adaptation of these techniques for infants and small children, the outlook for these patients has improved remarkably. In cases in which the patient has other serious illnesses or organ system failures and aggressive care is considered futile, the appropriate end-of-life recommendation may be for palliative care and comfort measures only. (See Quality Patient Outcomes box.) Quality Patient Outcomes Chronic Renal Failure • Sufficient calories and protein for growth maintained • Excretory demands made on the kidney are limited • Metabolic bone disease (osteodystrophy) minimal • Fluid and electrolyte disturbances managed • Hypertension managed • Growth retardation treated Nursing Care Management
ChatGPT said:
ChatGPT
Chronic Kidney Disease (CKD) is a condition characterized by the progressive loss of kidney function over time, often leading to end-stage renal disease (ESRD), where renal replacement therapy, such as dialysis or transplantation, becomes necessary. The following details outline various aspects of CKD, including etiology, pathophysiology, clinical manifestations, diagnostic evaluation, and therapeutic management.
Etiology
The causes of CKD vary by age. In children under 5 years, the most common causes include:
Congenital renal and urinary tract malformations (e.g., renal hypoplasia, dysplasia, obstructive uropathy).
Vesicoureteral reflux (VUR).
For children aged 5 to 15 years, glomerular and hereditary renal diseases become more prevalent, including:
Chronic pyelonephritis.
Chronic glomerulonephritis (CGN).
Hereditary conditions such as Alport syndrome and congenital nephrotic syndrome.
Renal vascular disorders, although they are less common.
Pathophysiology
In the early stages of CKD, children often remain asymptomatic despite some biochemical changes. As nephron damage progresses:
The surviving nephrons undergo hypertrophy to maintain fluid and electrolyte balance.
As nephrons continue to be destroyed, uremic symptoms develop, marked by the retention of waste products, water, sodium, and imbalances in electrolytes and acids.
Key biochemical disturbances include:
Waste Product Retention: Elevated serum creatinine and BUN indicate declining kidney function.
Water and Sodium Retention: Resulting in edema and hypertension, especially in ESRD.
Hyperkalemia: Typically occurs in later stages due to the kidneys' inability to excrete potassium.
Metabolic Acidosis: A hallmark of CKD, due to impaired excretion of hydrogen ions and bicarbonate reabsorption.
Calcium and Phosphorus Disturbances: Can lead to renal osteodystrophy due to complex alterations in mineral metabolism.
Clinical Manifestations
Early signs of CKD may include:
Fatigue and decreased energy.
Pallor due to anemia.
Hypertension.
Growth impairment, observable on growth charts.
As the disease progresses, additional symptoms can manifest, including:
Decreased appetite and altered activity levels.
Urinary output changes (e.g., enuresis).
Symptoms of uremia, such as nausea, vomiting, and itching.
Neurologic symptoms like confusion or seizures may occur in advanced stages.
Diagnostic Evaluation
Diagnosis typically involves:
Clinical Assessment: Identification of symptoms and history of renal disease.
Laboratory Tests: Assessment of renal function through serum creatinine and BUN levels, urine analysis, and imaging studies as needed.
GFR Classification: Determining the stage of CKD based on the glomerular filtration rate (GFR).
Therapeutic Management
Management goals include maximizing renal function, maintaining fluid and electrolyte balance, treating systemic complications, and promoting normal development and quality of life. Key components include:
Activity
Encourage unrestricted activity tailored to the child's stamina.
Facilitate school attendance and home tutoring if necessary.
Diet
Tailored to provide adequate calories and protein while minimizing renal excretory demands.
May involve restrictions on protein, phosphorus, and sodium based on disease stage and symptoms.
Monitoring and supplementation of vitamins and minerals (excluding excess fat-soluble vitamins).
Osteodystrophy Management
Address calcium and phosphorus imbalances through diet, phosphorus-binding agents, and vitamin D supplementation.
Acidosis Treatment
Alkalizing agents like sodium bicarbonate to correct metabolic acidosis.
Anemia Management
Erythropoietin therapy to stimulate red blood cell production, coupled with iron supplementation.
Hypertension Management
Lifestyle modifications with dietary sodium restriction, fluid management, and pharmacological treatment as needed.
Growth and Development Support
Focus on nutrition, correction of imbalances, and potentially growth hormone therapy.
Monitoring for Complications
Regular assessments for intercurrent infections and other complications, adjusting medication dosages based on renal function.
Nursing Care Management for Children with Chronic Kidney Disease (CKD)
Overview: Children with CKD rely heavily on medications and artificial interventions to maintain their health. The progressive nature of the disease and its management can impose significant stress on both the child and their family. Nursing care plays a vital role in supporting these families through various stages of the disease, from renal insufficiency to uremia, dialysis, or transplantation.
Multidisciplinary Approach: Effective care requires a coordinated effort from a multidisciplinary team, which may include:
Medical Professionals: Nephrologists, pediatricians
Nursing Staff: Nurses specializing in pediatric nephrology
Social Workers: To address emotional and financial concerns
Child Life Specialists: To provide support through play and education
Physical and Occupational Therapists: To help with physical development and daily activities
Dietitians: To manage dietary restrictions and nutritional needs
Psychologists/Psychiatrists: To support mental health
Family-Centered Care: The emotional and psychological impact of CKD on families can be profound. Nurses must recognize that while the ill child requires attention, family dynamics and priorities must also be considered. Families often face competing needs, such as work and the needs of siblings, which may influence their ability to comply with treatment schedules.
Key Considerations in Nursing Care:
Understanding Family Dynamics:
Each family's ability to cope with CKD is influenced by factors such as cultural background, socioeconomic status, and interpersonal relationships.
Nurses should facilitate open communication with families, helping them prioritize needs and navigate the complexities of care.
Support Through Treatment:
Frequent hospital visits and medical procedures can be overwhelming. Nurses should prepare families for what to expect and help them understand the treatment process.
Help families manage medication regimens, which can be complex and challenging to maintain over time.
Dietary Education:
Nurses should assist families in understanding dietary restrictions and modifications required for the child’s condition, including sodium and potassium limitations.
Educational resources should be provided to help parents read food labels and select appropriate meals.
Addressing Psychological Needs:
Encourage families to express their feelings about the diagnosis and treatment, and be alert for signs of anxiety and depression.
Offer support strategies and coping mechanisms to both children and parents, especially as they navigate the emotional challenges of chronic illness.
School Support:
Advocate for the child’s educational needs, ensuring they receive appropriate accommodations in school.
Collaborate with teachers and school staff to create a supportive learning environment, addressing attendance issues due to illness.
Financial Resources:
Educate families about available financial resources, including insurance coverage and community assistance programs.
Familiarize yourself with organizations like the National Kidney Foundation that provide educational materials and support for families.
Emotional Impact and Coping Strategies:
Children with CKD may experience noticeable physical changes that affect their self-image and interactions with peers. Common challenges include:
Body Image Issues: Encourage children to develop positive coping strategies and resilience in dealing with teasing or social stigma.
Behavioral Changes: Some children may exhibit regression in behavior due to stress and illness. Address these changes with supportive interventions.
19. Discuss laboratory findings associated with acute renal failure
Laboratory Findings Associated With Acute Renal Failure
Clinical ProblemMechanismClinical Considerations | ||
Azotemia | Ongoing protein catabolism | Lower rate of production in neonates and individuals with depleted protein stores. |
Elevated blood urea nitrogen levels | Significantly decreased excretion; increased in situations involving large amounts of necrotic tissue or extravasated blood. | |
Elevated plasma creatinine levels | Continued production; significantly decreased excretion; production less affected by other factors. More sensitive measure of intensity of azotemia; levels are low in neonates due to small muscle mass relative to size. | |
Metabolic acidosis | Continued endogenous acid production | Significantly decreased excretion; depletion of extracellular and intracellular fluid buffers; compensatory hyperventilation; opisthonos; major threat to life. |
Hyponatremia | Dilution of extracellular fluid | Decreased excretion of water; may develop cerebral signs. |
Hyperkalemia | Ongoing protein catabolism | Decreased excretion compounded by metabolic acidosis; most important electrolyte to consider in acute renal failure; may contribute to cardiac arrhythmia; with electrocardiogram changes, poses a major threat to life. Loss may occur from the gastrointestinal tract. |
Hypocalcemia | Associated with metabolic acidosis and hyperphosphatemia | During alkali therapy, may cause tetany. |
20. Describe therapeutic management and nursing indications for care related to oliguria, fluid and calories, hyperkalemia, hypertension, and other complications such as anemia, seizures, coma, cardiac failure, and pulmonary edema associated with acute renal failure.
1. Oliguria Management:
Therapeutic Management:
Monitor urine output carefully; define oliguria as less than 0.5 mL/kg/hour.
Assess for signs of fluid overload (edema, hypertension).
Administer diuretics (e.g., furosemide) if prescribed, based on the patient's fluid status and renal function.
Consider dialysis if oliguria persists and leads to complications.
Nursing Indications:
Maintain strict intake and output records.
Weigh the child daily to monitor fluid status.
Observe for symptoms of dehydration or fluid overload.
2. Fluid Management:
Therapeutic Management:
Calculate fluid requirements based on the child’s weight, age, and clinical status.
Administer fluids cautiously to avoid overload; consider fluid restrictions if necessary.
Nursing Indications:
Monitor vital signs for signs of fluid overload (e.g., increased blood pressure, tachycardia).
Assess lung sounds and respiratory status to detect pulmonary edema.
Provide education to families about fluid restrictions and signs of fluid imbalance.
3. Caloric Management:
Therapeutic Management:
Provide a diet high in carbohydrates and fats, with restricted protein intake as prescribed.
Monitor nutritional status and weight to ensure adequate caloric intake.
Nursing Indications:
Collaborate with a dietitian to develop meal plans.
Educate families on appropriate food choices and reading food labels.
Monitor for signs of malnutrition or growth failure.
4. Hyperkalemia Management:
Therapeutic Management:
Monitor serum potassium levels regularly; normal range is 3.5–5.0 mEq/L.
Administer medications such as calcium gluconate, insulin with glucose, or sodium bicarbonate to stabilize cardiac membranes and lower potassium levels.
Consider dialysis if potassium levels are dangerously high or if other treatments are ineffective.
Nursing Indications:
Assess the child for signs of hyperkalemia (e.g., muscle weakness, ECG changes).
Educate families about potassium-restricted diets and foods to avoid (e.g., bananas, oranges, potatoes).
5. Hypertension Management:
Therapeutic Management:
Monitor blood pressure regularly; normal range varies by age.
Administer antihypertensive medications as prescribed (e.g., ACE inhibitors, calcium channel blockers).
Nursing Indications:
Educate families on the importance of regular blood pressure monitoring.
Encourage lifestyle modifications such as dietary changes (low sodium).
6. Management of Other Complications:
Anemia:
Therapeutic Management: Administer erythropoiesis-stimulating agents (e.g., epoetin alfa) and iron supplements as needed.
Nursing Indications: Monitor hemoglobin and hematocrit levels; educate families on signs of anemia.
Seizures and Coma:
Therapeutic Management: Ensure seizure precautions; administer anticonvulsants as prescribed.
Nursing Indications: Monitor neurological status; assess for any triggers or changes in consciousness.
Cardiac Failure:
Therapeutic Management: Monitor for signs of heart failure; administer diuretics and medications to manage heart function.
Nursing Indications: Monitor vital signs and fluid status closely.
Pulmonary Edema:
Therapeutic Management: Administer oxygen as needed; consider diuretics to manage fluid overload.
Nursing Indications: Monitor respiratory status and lung sounds for signs of fluid accumulation.
21. Describe the etiology of CRF.
Causes of CKD by Age Group
Before Age 5 Years:
Congenital Renal and Urinary Tract Malformations:
Renal Hypoplasia and Dysplasia: These are conditions where the kidneys are underdeveloped or malformed, leading to impaired kidney function.
Obstructive Uropathy: This occurs when there is a blockage in the urinary tract that impedes the flow of urine, potentially causing kidney damage.
Vesicoureteral Reflux (VUR): A condition where urine flows backward from the bladder into the ureters and sometimes into the kidneys, which can lead to recurrent urinary tract infections and kidney damage.
Ages 5 to 15 Years:
Glomerular Diseases:
Chronic Pyelonephritis: A recurring kidney infection that can lead to scarring and loss of kidney function.
Chronic Glomerulonephritis (CGN): A progressive kidney disease that affects the glomeruli (filtering units of the kidney), resulting in inflammation and scarring.
Glomerulonephropathy Associated with Systemic Diseases:
Anaphylactoid Purpura (Henoch-Schönlein Purpura): A small-vessel vasculitis that can affect the kidneys.
Systemic Lupus Erythematosus (SLE): An autoimmune disease that can lead to kidney inflammation and damage.
Hereditary Renal Diseases:
Hereditary Nephritis: A genetic disorder affecting the glomeruli, often leading to progressive kidney failure.
Congenital Nephrotic Syndrome: A genetic condition causing excessive protein loss in urine and resulting in kidney failure.
Alport Syndrome: A genetic condition characterized by kidney disease, hearing loss, and eye abnormalities.
Polycystic Kidney Disease (PKD): A hereditary disorder causing numerous cysts to grow in the kidneys, leading to kidney enlargement and dysfunction.
Less Common Causes:
Renal Vascular Disorders:
Hemolytic Uremic Syndrome (HUS): A condition often triggered by infections that can lead to kidney failure.
Vascular Thrombosis: The formation of a blood clot in a renal vein can disrupt kidney function.
Cortical Necrosis: A rare condition characterized by the death of the kidney cortex, often due to severe hypotension or other critical illnesses.
22. Discuss indications for hospice care, dialysis, and renal transplantation.
Indications for Hospice Care, Dialysis, and Renal Transplantation
Managing chronic kidney disease (CKD) and its complications in children requires careful consideration of various treatment modalities, including hospice care, dialysis, and renal transplantation. The choice of treatment often depends on the child's condition, the nature of the renal failure, and the family's preferences and circumstances.
1. Indications for Hospice Care
Hospice care is a compassionate approach for children with terminal illnesses, including those with advanced CKD, who are not responding to curative treatments. Key indications for hospice care include:
End-Stage Renal Disease (ESRD): When the child is in the final stages of CKD and dialysis is not an option or is no longer effective.
Palliative Needs: Focus on relieving symptoms and improving the quality of life rather than prolonging life. This includes pain management and psychological support.
Family Considerations: Families may choose hospice care to provide a supportive environment that addresses emotional and spiritual needs, allowing them to spend quality time together in the child’s final days.
Non-Compliance with Treatment: Children who refuse dialysis or have severe comorbidities that complicate treatment may benefit from hospice care.
Limited Life Expectancy: When a child’s prognosis is poor, with a life expectancy of typically six months or less, hospice care can provide supportive measures and comfort.
2. Indications for Dialysis
Dialysis is indicated for children with acute or chronic renal failure when conservative management is insufficient. The decision to initiate dialysis is based on several criteria:
Acute Conditions: Dialysis may be required in acute renal failure due to:
Severe metabolic acidosis.
Severe electrolyte imbalances (e.g., hyperkalemia, severe hypernatremia).
Severe volume overload (e.g., congestive heart failure with fluid overload).
Acute poisoning or toxic ingestions.
Tumor lysis syndrome in oncology patients.
Chronic Conditions: For children with chronic kidney disease, indications for dialysis include:
Biochemical abnormalities: Elevated blood urea nitrogen (BUN), hyperphosphatemia, and high potassium levels that are life-threatening.
Clinical manifestations: Deterioration of central nervous system function, congestive heart failure unresponsive to medical management, growth failure, severe osteodystrophy, and insufficient caloric intake.
Inability to perform daily activities: Indicating a significant impact on quality of life.
Dialysis is typically initiated when the child is in end-stage renal failure (ESRF) unless urgent intervention is needed in acute settings.
3. Indications for Renal Transplantation
Renal transplantation is the preferred treatment for children with end-stage renal disease when feasible, offering the best potential for long-term survival and quality of life. Indications for renal transplantation include:
End-Stage Renal Disease: Children with irreversible kidney failure who are not likely to benefit from dialysis or have a poor prognosis with dialysis alone.
Age and Growth Considerations: Transplantation is often more beneficial for younger children, as it allows for improved growth and development compared to remaining on dialysis.
Good Overall Health: Candidates must be in good general health to withstand the surgery and the immunosuppressive therapy that follows transplantation.
Absence of Contraindications: Certain medical conditions or factors may disqualify a child from being a transplant candidate, including active infections, malignancies, or severe comorbidities.
Family Support and Compliance: A strong support system and the ability to adhere to the postoperative regimen are critical for the success of a transplant.
23. Identify the indications and nursing care management for renal replacement therapy via hemodialysis, peritoneal dialysis, and continuous venovenous hemofiltration.
Hemodialysis:
Indications: Preferred for acute conditions like life-threatening hyperkalemia or poisoning. Suitable for children living close to a dialysis center, especially those who are noncompliant with PD.
Peritoneal Dialysis
Indications: Effective for acute conditions and chronic management in children, particularly those who prefer fewer dietary restrictions and gentler processes. It is often performed at home.
Continuous Venovenous Hemofiltration (CVVH)
Indications:
Acute Kidney Injury (AKI): Especially in critically ill patients who require continuous renal replacement therapy.
Fluid Overload: Patients who cannot tolerate rapid fluid removal via traditional hemodialysis.
Severe Metabolic Disturbances: Conditions like hyperkalemia and metabolic acidosis needing gradual correction.
Pediatric Patients: Particularly those with fluid overload from surgical procedures without severe biochemical abnormalities.
24. Discuss renal transplantation issues, including selection of donor tissue, immunosuppression, and signs of rejection.
Kidney Transplantation: Overview and Nursing Management
Kidney transplantation is the preferred form of renal replacement therapy for children with end-stage renal disease (ESRD). It offers a better quality of life compared to dialysis, allowing children to return to a more normal lifestyle.
Sources of Donor Kidneys
Living Donor: Typically a family member (parent, grandparent, sibling).
Deceased Donor: Kidneys are procured from individuals who have consented to organ donation after brain death.
Recipient Selection Criteria
Criteria for selecting transplant candidates vary by center, with no strict uniformity. Factors affecting candidacy may include:
Mental Health: Emotional stability and ability to adhere to medication regimens are critical.
Medical Conditions: Recipients must be cancer-free for a specified period if they previously had malignancies.
Infections: Any generalized infections must be treated prior to transplantation.
Bladder Capacity: Adequate bladder function is essential for transplant success.
Transplant Procedure
The transplanted kidney is usually placed in the extraperitoneal space (anterior iliac fossa).
Surgical connections:
Renal Artery: Anastomosed to the internal iliac or hypogastric artery.
Renal Vein: Anastomosed to the hypogastric vein.
Ureter: Implanted into the bladder or anastomosed to the recipient's ureter.
Immunological Considerations
Tissue Compatibility: The closer the genetic match, the better the chances for graft survival. Identical twins are the best match, followed by siblings, parents, and grandparents.
Immunosuppressant Therapy: Essential to prevent rejection. Common regimens include:
Prednisone: An anti-inflammatory that suppresses immune responses.
Tacrolimus: Reduces T-cell activation.
Mycophenolate: Inhibits lymphocyte proliferation.
Monitoring for Rejection:
Hyperacute Rejection: Occurs immediately post-transplant and is irreversible.
Acute Rejection: Can occur days to months post-transplant; may be treated with corticosteroids or monoclonal antibodies.
Chronic Rejection: Characterized by gradual decline in kidney function; often irreversible.
25. Identify common defects of the genitourinary tract in infants and young children.
1. Phimosis
Definition: A condition where the preputial opening of the foreskin cannot be retracted over the glans penis.
Normal Development: Typically normal in infants; often resolves naturally as the child grows.
Complications: Can lead to urinary obstruction or balanitis (inflammation/infection of the foreskin).
Treatment: Steroid cream application or circumcision for severe cases.
Nursing Care: Maintain proper hygiene, avoid forcible retraction to prevent scarring, and monitor for signs of paraphimosis (emergency situation).
2. Hydrocele
Definition: Accumulation of fluid in the scrotum, leading to painless swelling.
Types:
Communicating: Fluid communicates with the peritoneal cavity (often resolves spontaneously).
Noncommunicating: No connection with the peritoneum (may require treatment if symptomatic).
Management: Surgical intervention for persistent communicating hydroceles after age 1; usually outpatient procedures.
Nursing Care: Educate parents on post-operative care, swelling management, and activity restrictions.
3. Cryptorchidism
Definition: Failure of one or both testes to descend into the scrotum.
Prevalence: More common in premature infants; spontaneous descent often occurs in the first 6 months.
Complications: Increased risk of inguinal hernia, testicular torsion, infertility, and testicular cancer.
Diagnosis: Physical examination to differentiate between nonpalpable and retractile testes; laparoscopic surgery for further evaluation.
Therapeutic Management: Orchiopexy recommended if no descent occurs by 6 months; typically performed between 6-24 months.
Nursing Care: Postoperative care to prevent infection, manage pain, and educate on testicular self-examination.
4. Hypospadias
Definition: Congenital abnormality where the urethral opening is located on the ventral side of the penis.
Incidence: Affects approximately 1 in 300 males, with higher recurrence in first-degree relatives.
Severity: Based on the location of the urethral opening and associated penile curvature (chordee).
Associated Conditions: Up to 10% of infants with hypospadias may also have cryptorchidism.
Management: Surgical correction, typically between 6-18 months of age.
Nursing Care: Pre- and postoperative education, monitoring for infection, and ensuring proper wound care.
Epispadias and Exstrophy Complex (EEC)
Overview
Bladder exstrophy is a severe congenital defect affecting the musculoskeletal, urinary, reproductive, and sometimes gastrointestinal systems. It is part of the exstrophy-epispadias complex (EEC), which includes:
Epispadias: The least severe condition where the urethra fails to close properly, leading to an exposed dorsal urethra.
Bladder Exstrophy: A more severe condition characterized by an "inside-out" bladder, where the inner surface is exposed through an abdominal wall defect. Associated anomalies may include diastasis of the symphysis pubis, low-set umbilicus, anteriorly displaced anus, genital defects, and inguinal hernia.
Cloacal Exstrophy: The most severe form, involving both bladder and intestinal exstrophy, anal atresia, omphalocele, hypoplasia of the colon, and genital anomalies. It is associated with spinal dysraphism and renal abnormalities.
Prevalence
Cloacal Exstrophy: 1 in 131,579 live births, more common in females.
Bladder Exstrophy: 2.07 per 100,000 live births, nearly twice as common in males than females.
Pathophysiology
The etiology of bladder exstrophy is linked to a complex embryologic defect in abdominal wall development. This condition leads to various genital and urinary tract anomalies:
Males: Epispadias, upward curvature of the penis, cryptorchidism, and potential inguinal hernias.
Females: Epispadias, bifid clitoris, small labia minora, and a shortened vagina. Cloacal exstrophy may also result in a bifid or duplicated uterus and significant genital anomalies.
26. Describe disorders of sex development.
Disorders of Sex Development (DSD)
Definition and Presentation
Disorders of Sex Development (DSD) refer to a group of conditions in which there is a discrepancy between the chromosomal, gonadal, and anatomical sex of an individual. Infants with DSD may exhibit ambiguous genitalia at birth, making it difficult to assign a clear gender. Common presentations of DSD include:
Bilateral Cryptorchidism: Undescended testicles in a male infant.
Perineal Hypospadias with Bifid Scrotum: An abnormality where the urethra opens on the underside of the penis.
Clitoromegaly: Enlargement of the clitoris.
Posterior Labial Fusion: Fusion of the labia, often observed in females.
Phenotypic Female Appearance with Palpable Gonad: A female external appearance but with an undescended testis.
Hypospadias with Unilateral Nonpalpable Gonad: A urethral opening that is not at the tip of the penis alongside a non-visible testicle.
Additionally, chromosomal disorders such as Turner syndrome (45, XO) and Klinefelter syndrome (47, XXY) are classified as DSDs, even though they may not present with ambiguous genitalia.
Pathophysiology
Normal sexual differentiation begins at approximately 7 weeks of gestation, with the presence of a Y chromosome leading to testicular development. Both male (XY) and female (XX) fetuses initially develop similar reproductive structures, and various genes are involved in this differentiation. Mutations in these genes can result in different forms of DSD. The majority of congenital malformations in genitalia are attributed to:
Androgen Deficiency: Typically affects XY individuals.
Androgen Excess: Common in XX individuals.
Some cases may not have a discernible endocrine cause. Evaluation typically begins with karyotyping and an assessment of adrenal and gonadal function, allowing classification into:
Virilized XX (XX DSD)
Undervirilized XY (XY DSD)
Mixed sex chromosome patterns
Therapeutic Management
Congenital adrenal hyperplasia (CAH) is the most prevalent cause of ambiguous genitalia, which can lead to serious complications, including life-threatening adrenal insufficiency. Timely evaluation is critical. Initial laboratory tests include measuring 17-hydroxyprogesterone and performing a karyotype with an SRY gene probe. Monitoring for signs of adrenal insufficiency (e.g., hypoglycemia, hyponatremia, hyperkalemia) is vital, and appropriate fluid and electrolyte replacement may be necessary. Additional testing, including imaging studies like pelvic and abdominal ultrasound, may be conducted to assess gonadal structures.
Obstructive Uropathy
Definition
Congenital urinary obstruction is characterized by impaired urinary flow that may limit normal renal development. This condition encompasses a wide range of disorders and is one of the most common issues affecting the urinary tract in children. Obstruction may be congenital or acquired, unilateral or bilateral, and can be complete or incomplete.
Pathophysiology
Obstruction can occur at any level within the urinary tract and leads to dilation of the urinary structures above the obstruction. For example:
Ureteropelvic Junction Obstruction (UPJO): The most common site of obstruction, where the ureter meets the renal pelvis.
Posterior Urethral Valves (PUVs): Membranes obstructing the urethra, primarily affecting males.
Congenital Megaureter and Ureterocele: Abnormalities of ureter development that can cause obstruction.
The severity and timing of the obstruction during kidney development can lead to various outcomes, including renal dysplasia and potential dysfunction.
Clinical Manifestations
Symptoms of obstructive uropathy vary based on the location and severity of the obstruction. Common presentations include:
Prenatal Findings: Hydronephrosis detected on fetal ultrasound.
Postnatal Symptoms: Abdominal mass, urinary tract infection, or signs of renal failure. In males, PUVs can lead to respiratory distress or sepsis due to associated oligohydramnios.
Pain and Infection: Conditions like UPJO may present with abdominal or flank pain and nausea. Ureterocele or megaureter may present with urinary tract infections or hematuria.
Obstructive uropathy can also lead to lower urinary tract symptoms, including urinary stream abnormalities, frequency, urgency, and nocturia.
 Knowt
Knowt