Unit 3: Nuclear Chemistry
Atoms
Atoms are spherically shaped with a tiny, dense, positively charged nucleus, contain protons and neutrons. Most of an atom consists of fast-moving negatively charged electrons traveling through empty space surrounding the nucleus. The electrons occupy different energy levels, similar to the rungs on a ladder.
Proton | Neutron | Electron | |
Symbol | p+ | n0 | e- |
Location | Nucleus | Nucleus | Electron cloud |
Electrical Charge | Positive | Neutral | Negative |
Relative Mass | 1 amu | 1 amu | 0 amu |
The number of protons in an atom identifies it as an atom of a particular element
The number of protons is called the atomic number
Elements are arranged on the Periodic Table by increasing atomic number
On the Periodic Table, each different element is shown in a box
Atomic number = protons = electrons
Mass number = protons + neutrons
Isotopes
All atoms of a particular element have the same number of protons and electrons but the number of neutrons may vary. Atoms with the same number of protons but different number of neutrons are called isotopes.
Isotopes differ in mass but have the same chemical behavior. To identify the various isotopes of an element, the mass number is added after the element’s name. It represents the sum of the protons and neutrons in the nucleus.
Number of Neutrons = Mass number - atomic number
For example, a carbon atom with 6 protons and 6 neutrons is called carbon-12
Scientists
Democritus (460-370 BC)
The first person to propose the idea that matter was not infinitely divisible. He believed matter was made up of tiny individual particles called atomos. He believed that atoms could not be created, destroyed, or further divided.
Matter is composed of empty space through which atoms move
Atoms are solid, homogeneous, indestructible, and indivisible
Different kinds of atoms have different sixes and shapes
The differing properties of matter are due to the size, shape, and movement of atoms
Apparent changes in matter result from changes in the groups of atoms and not from changes in the atoms themselves
His ideas were met with criticism from other philosophers.
John Dalton (1766-1844)
Dalton’s Atomic Theory:
All matter is made of indivisible and indestructible atoms
Atoms of a given element are identical in their physical and chemical properties
Atoms of different elements have different physical and chemical properties
Atoms of different elements can combine in simple whole-number ratios to form chemical compounds
Atoms cannot be subdivided, created, or destroyed when they are combines separated, or rearranged in chemical reactions
Dalton’s theory explains the law of conservation of mass: atoms of different elements combine to form chemical compounds
Not entirely accurate → Weakness was his belief that the atom was indivisible
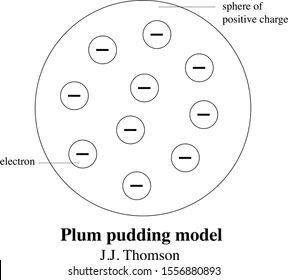
J.J. Thomson (1856-1940)
Thomson’s experiments concluded that there were tiny, negatively charged particles called electrons. Thomson proposed that tiny negatively charged electrons must be embedded in a cloud of positive charge, which he known as the “Plum Pudding Model.”
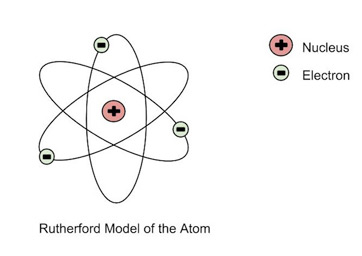
Ernest Rutherford (1871-1937)
A student of J.J. Thomson, Rutherford performed the “gold-foil experiment” which ultimately disproved J.J. Thomson’s theory. With a new model, called the Rutherford model, the electrons orbit around the very small and dense nucleus.
Ions
An atom is an electrically neutral particle composed of electrons, neutrons, and protons. Usually, the number of protons equal the number of electrons. If an atom gains or loses electrons, it is called an Ion.
Cation: A positively charged ion that has lost an electron
There are more protons than electrons, which makes it positively charged
ex. Cl2+, Ca+
Anion: A negatively charged ion that gained an electron
There are more electrons than protons, which makes it negatively charged
ex. S2-, O-
Nuclear Chemistry
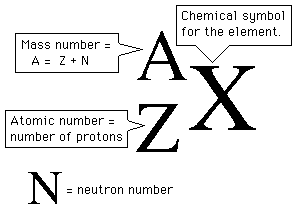
Mass Defect: Difference between the mass of an atom and the mass of its individual particles
Nuclear Binding Energy: Energy released when a nucleus is formed from nucleons. High binding energy means a more stable nucleus
E=mc2
E: Energy (J)
m: mass defect (kg)
c: speed of light (3.00 × 108 m/s)
Types of Radiation:
Transmutation: the changing of one element into another by radioactive decay
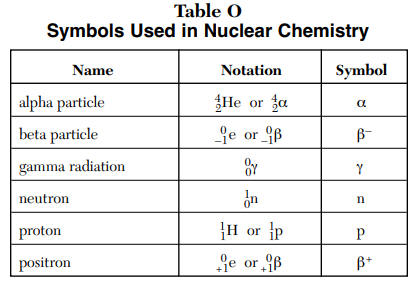
Alpha
Lowest penetration power: stopped by paper, clothes, skin
Beta
Relative penetration power: stopped by aluminium foil
Positron
Relative penetration power: stopped by aluminium foil
Gamma
Greatest penetration power: stopped by concrete, lead
Electron Capture
Relative penetration power: stopped by aluminium foil
Neutron
Relative penetration power: stopped by water
Half-Life
Half life is the time required for the number of radioactive nuclides to decay to half of the original value
Parent isotope → Daughter isotope
Shorter half life means it is more unstable
Expressed as t1/2
Formula: mf = mi(1/2)n
mf = final mass
mi = initial mass
n = number of half lives
n = total time / time in one half life
 Knowt
Knowt