AP Environmental Science 9.1-9.2 - Ozone Depletion
What is the Ozone Shield?
Ozone Shield: a natural process that filters ultraviolet (UV) radiation before it reaches the lower atmosphere.
- The layer of ozone gas (O3) in the upper stratosphere that screens out harmful ultraviolet radiation from the sun.
- If the full amount of ultraviolet radiation falling on the stratosphere reached Earth’s surface; it is doubtful that any life could survive.
Ozone Depletion
How does Ozone Depletion Occur?
Overview
- CFCs (clorofluorocarbons) emitted into atmosphere; they’re stable, move from troposphere to stratosphere
- UV light breaks off chlorine molecule (Cl) from the CFC particle
- Cl acts as a catalyst to break down ozone (O3)
- catalyst – promotes a chemical reaction without itself being used up in the reaction
- Shifts equilibrium of oxygen / ozone reaction
Summary of Reactions
- Cl₃CF (example CFC) + UV → Cl+CCl₂
- Cl+O₃ → ClO + O₂
- ClO + O → Cl + O₂
- This cycle repeats itself several times
“From Dream Chemicals to Nightmare Chemicals”
- Thomas Midgley, Jr. A General Motors chemist, discovered the first chlorofluorocarbon (CFC) in 1930.
- Family of highly useful CFCs – trichlorofluoromethane and dichlorodifluoromethane (AKA; freons)
- Stable, odorless, nonflammable, nontoxic, and noncorrosive
- Used in air conditioners, refrigerators, aerosol spray cans, cleaners for electronic parts, sterilants for hospital instruments, fumigants for granaries, bubbles in plastic foam used for packaging.
Consequences of Ozone Depletion
Humans
- Increase in skin cancer & cataracts, especially in the southern hemisphere
- More ozone near earth’s surface, produced in photochemical smog – lung problems, suppressed immune response, cancer
Threat of Ozone Depletion
- Radiation from the sun includes ultraviolet (UV) radiation; UVA and UVB
- UV radiation penetrates the atmosphere and is absorbed by biological tissues damaging protein and DNA molecules at the surfaces of all living things (sunburn).
- Most of the dangerous UVB radiation (over 99%) is absorbed by ozone in the stratosphere.
Other Organisms
- Primary Producers:
- Reduction in phytoplankton
- Lower crop yields
- Decline in forest productivity
- Animals:
- Species disruption through increased exposure to UV-B radiation
- Disruption of food chain
Effect of Ozone Depletion
Ozone Depleting Chemicals
- Chlorofluorocarbons (CFCs)
- Halons: fire extinguishers
- Methyl bromide: fumigant
- Carbon tetrachloride: cheap, highly toxic solvent
- Methyl chloroform: cleaning solvent-clothes & metals
- Hydrogen chloride; U.S. space shuttles
Ozone Hole
- Seasonal thinning of the ozone layer has resulted at the poles, especially in the southern hemisphere
- Recent models suggest the hole might not get larger
Why is there Seasonal Thinning of Ozone Over the Poles?
- In 1984, researchers discovered 40-50% of the ozone in the upper stratosphere over Antarctica was being destroyed during the antarctic spring and early summer (Sept.-Dec.)
- In 2000, ozone thinning above Antarctica was the largest ever and covered an area three times the size of the continental U.S. (11 million square miles)
- Measurements indicate that CFCs are the primary culprits.
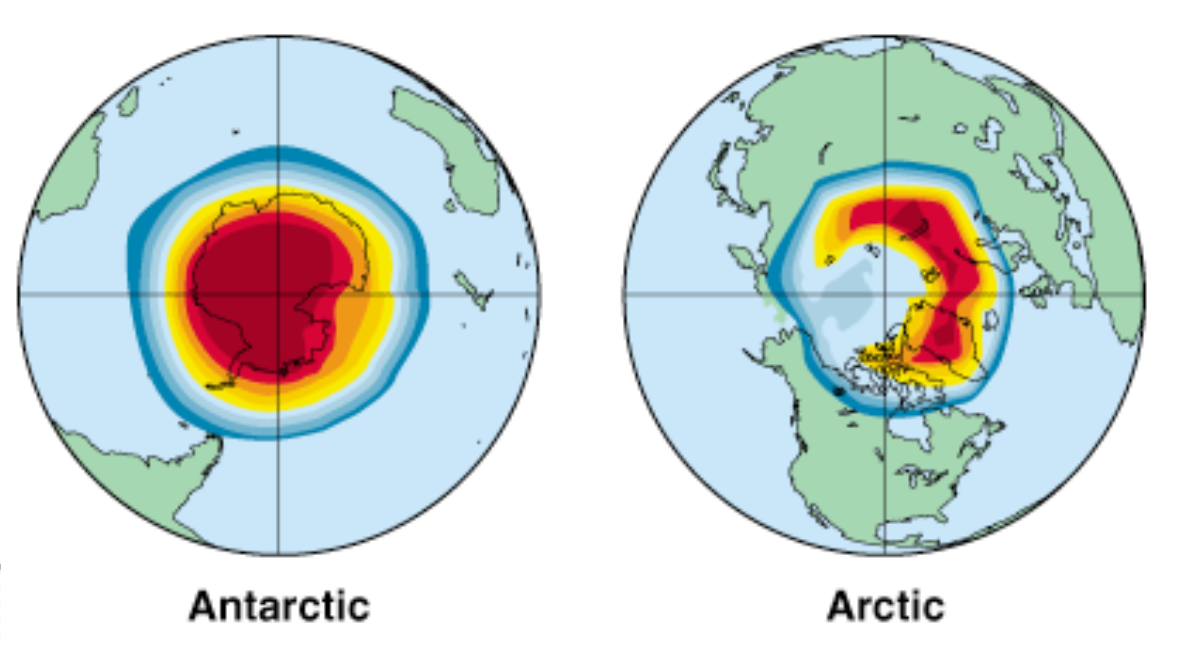
Ozone Loss
Projected total ozone loss, averaged over 2010-2019, during September for the Antarctic (left) and during March for the Arctic (right). Dark red represents ozone depletion of 54% or more; light blue, 18-30%; dark blue, 6-12%.
Solutions to Ozone Depletion
General
- Phase out use of ozone–depleting chemicals (halons, CFCs, methyl chloroform, methyl bromide)
- Phase in use of CFC substitutes [non–halogen aerosol propellants, hydrochlorofluorocarbons (HCFCs), hydrofluorocarbons (HFCs), hydrocarbons (HCs), ammonia, water & steam, terpenes, helium]
International Agreements
- Montreal Protocol (1987)
- Cut emission of CFCs by 35% by 2000
- London (1990) and Copenhagen (1992)
- Accelerate phase-out of other key ozone-depleting chemicals
World Meteorological Organization
- Continued depletion for several decades
- 11-20 year time lag between when CFCs are released into the atmosphere and when they actually reach the stratosphere.
- Persistence for decades
- Return to 1980 levels by about 2050 and to 1950 levels by about 2100.
- International agreements are followed
- No major volcanic eruptions
- Restoring the ozone layer may lead to an increase in global warming
- Ozone depletion has been cooling the troposphere
- Disguise as much as 30% of global warming caused by our greenhouse gas emissions.
How Can We Protect the Ozone Layer?
- Technofixes
- Huge radio-controlled blimps to form an electrical curtain.
- Lasers blasting CFCs out of the atmosphere before they reach the stratosphere.
- Montreal Protocol
- Phase-out CFC emissions
- Copenhagen Protocol
- Phase-out CFC emissions and other ozone deleters