Rules of Metabolic Reactions
Understand why ATP hydrolysis is exergonic
\n @@ATP hydrolysis is exergonic because the entropy of the product molecules is higher than that of the reactants@@, and because there is a large drop in potential energy when ATP is hydrolyzed to form ADP and Pi. The change in potential energy occurs because the new bonds formed in the products are much stronger than those in ATP.
\
Predict the impact of [substrate] vs [product] on a reaction’s rate
\n
==Increasing substrate concentration also increases the rate of reaction to a certain point==. Once all of the enzymes have bound, any substrate increase will have no effect on the rate of reaction, as the available enzymes will be saturated and working at their maximum rate.
\
Product
\
Predict and explain the impact of temperature, concentration, surface area on rates of reactions.
- Temperature: An increase in temperature typically increases the rate of reaction. An increase in temperature will raise the average kinetic energy of the reactant molecules. Therefore, a greater proportion of molecules will have the minimum energy necessary for an effective collision.
- Concentration: ==Increasing the concentration of one or more reactants will often increase the rate of reaction.== This occurs because a higher concentration of a reactant will lead to more collisions of that reactant in a specific time period.
Surface area: Large surface area helps more reactant molecules to undergo reaction at the same time to convert into the product, as the number of molecules undergoing reaction at the same time increases, the rate of reaction increases.
Gibbs free energy vs reaction progress
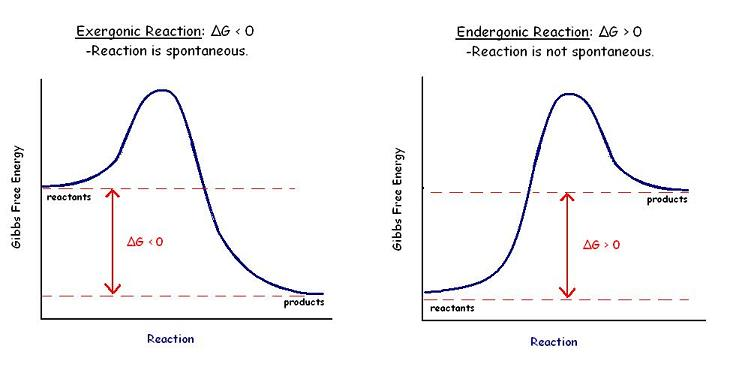
%%The Gibbs free energy graph shows whether or not a reaction is spontaneous%%-- whether it is exergonic or endergonic. Therefore, if the reaction goes from higher free energy to lower free energy, there will be a negative ΔG, and the reaction will be spontaneous. A positive ΔG indicates that the reaction is endergonic, or that it requires energy to go from reactants to products.
\
\