Diffusion & Osmosis
Solutions
Before you can understand diffusion and osmosis, you need to understand solutions. Solutions have both a solute and a solvent. The solute is the substance that is dissolved within the solution and the solvent is the substance doing the dissolving.
- For example, if you make sweet tea, you need to dissolve sugar in the tea. The sugar is the solute and the tea is the solvent.
You also need to understand the concentration of solutes in solutions to understand diffusion (and osmosis). The more solutes you have in a solution, the higher the concentration of the solution.
- Back to the last example, if you add 2 cups of sugar to your tea, that sweet tea solution will be more concentrated than if you only added 1 cup of sugar to your tea.
A solution with a low concentration of solutes is considered dilute and a solution with a high concentration of solutes is considered concentrated.
In living organisms, we have something called a concentration gradient. This occurs when one side of a membrane has a different concentration of solutes than the other side. Typically, a cell has a higher concentration of solutes because cells are full of important nutrients to help cellular processes run smoothly. Outside the cell is usually less concentrated because there is so much space for the solutes to spread out.
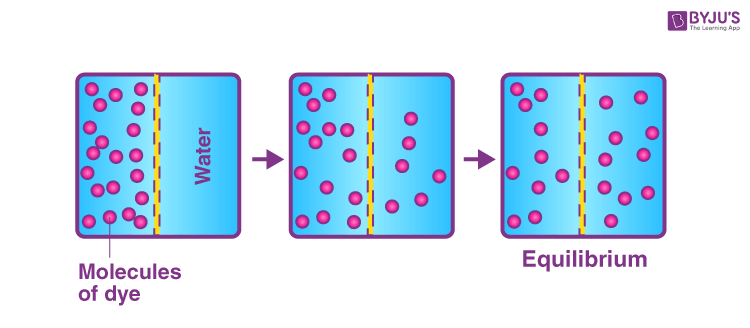
Particles do not have feelings or opinions, but they are always trying to establish equilibrium. Equilibrium is when the concentrations on both sides of the membrane are the same. The root word equ means “the same” or “equal.” So, wherever a permeable membrane is present, the concentration of solutes on either side of the membrane will continually try to establish equilibrium.
Brownian Motion
- Particles are always moving. In fact, the particles of the table that your computer is sitting on right now are moving. Because they are particles of solids, they aren’t moving very fast and are simply vibrating in place, but they are moving. Particles do not, however, choose where they want to go. They do not have a GPS and they cannot take directions. They simply move randomly around their container or the area in which they are located. This is known as Brownian motion.
- Because particles move randomly, they are continually bumping into one another. As these particles bump into each other, they bounce off of each other and move away from each other, only to hit another particle, and repeat the collision process. If molecules have space, this random Brownian motion will cause particles to naturally move away from each other. If particles are in a very large area, they will bump into each other and move away from each other in opposite directions as far as they can go. This tendency of particles to randomly move away from each other is how particles move “down” a concentration gradient, establishing equilibrium.
Diffusion
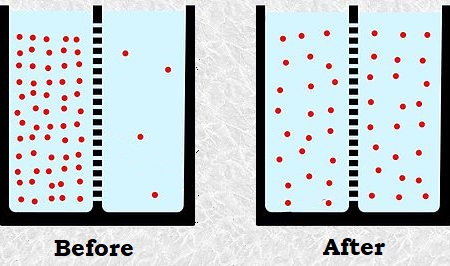
Diffusion is a cellular process that moves particles from an area of high particle concentration to an area of low particle concentration.
Because particles naturally "spread out" (moving from a more crowded space to a less crowded space) due to Brownian motion, diffusion happens naturally without any energy input. Because no energy is required for diffusion to occur, it is known as passive transport, which is particle movement in and out of the cell that requires no energy.
Osmosis
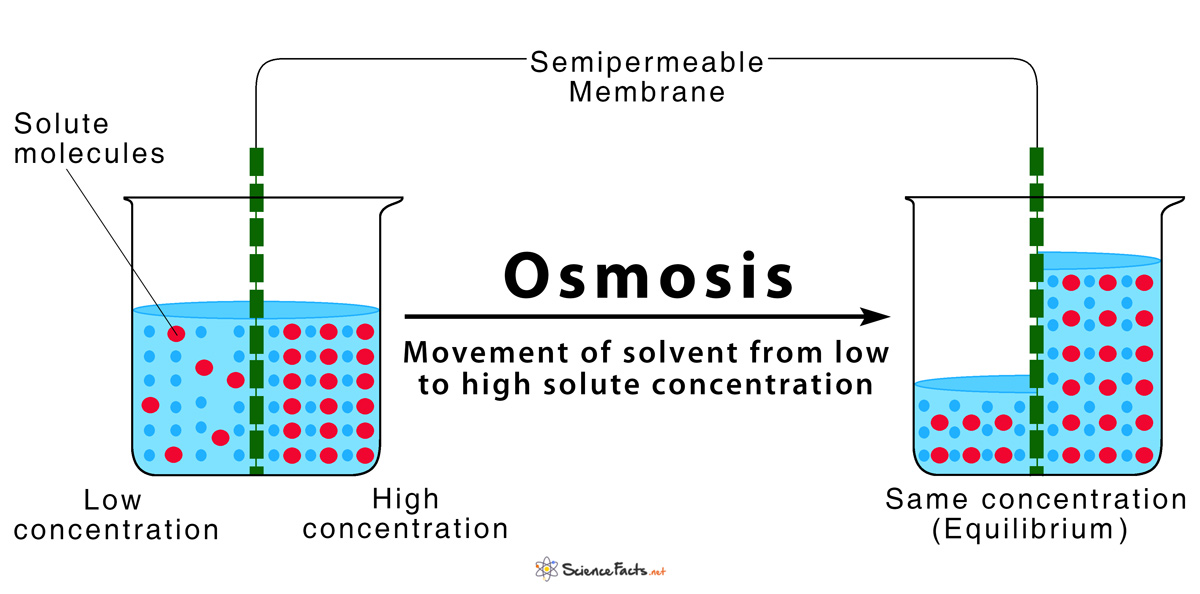
Osmosis is similar to diffusion except water is moving from an area of low particle concentration to an area of high particle concentration.
Like diffusion, osmosis does not require any energy; it happens naturally, so it is also a type of passive transport. The difference between osmosis and diffusion is that diffusion is the movement of particles and osmosis is the movement of water. During osmosis, the water is moving to establish equilibrium, not the particles, so the water will move to areas where there are more solutes to dilute the solute concentration.
The goal of both osmosis and diffusion is an equilibrium — an equal concentration of solutes on both sides of the concentration gradient.
 Edited: 05 October 2022
Edited: 05 October 2022