Chapter 13: Species Identification
13.1: General Considerations
Types of Antibodies
- An antihuman antibody that is used in the identification of human samples can be made by introducing human serum into a host animal, which then produces specific antibodies against the human serum proteins.
- Antibodies produced from different species of host animals may produce variations in the characteristics of reactions.
- Albumin: A protein that plays important roles in the maintenance of the vascular circulating fluid and the transportation of various substances such as nutrients, hormones, and metabolic products.
- Hemoglobin: An oxygen-transport protein that is found in erythrocytes.
- Purified Hemoglobin: Can be used to generate monoclonal and polyclonal antihuman Hb antibodies.
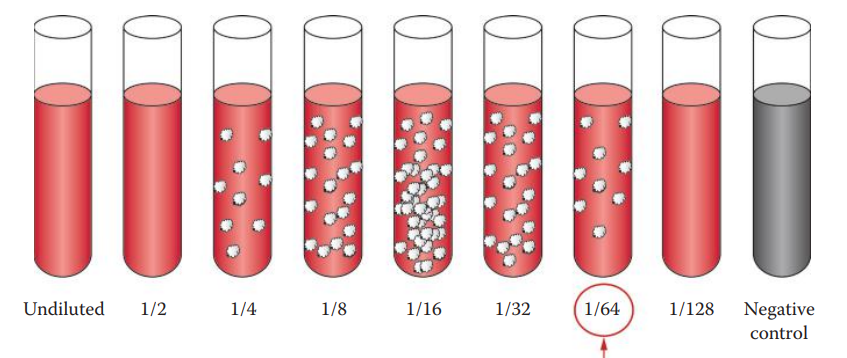
Titration of Antibodies
An extreme excess of antigen or antibody concentrations can inhibit secondary reactions.
The prozone and postpone phenomena must be considered, and the concentrations of antigen and antibody must be carefully determined for forensic serology assays.
Quality-control procedures can be used to estimate the amount of a specific antibody that is present, often via titration.
To titrate an antiserum, a series of dilutions are made and each dilution is then tested for activity using precipitation or agglutination methods.
Titer: The reciprocal of the highest dilution giving a positive reaction.
Antibody Specificity
- The specificity of the antihuman antibody must be tested.
- Most anti-human antibodies usually have cross-reactivity with higher primates. This is not a great concern because crimes involving nonhuman primates are very rare.
- The anti-human antibody must not cross-react with other commonly encountered animals.
Optimal Conditions for Antigen–Antibody Binding
- Stronger inhibition is usually observed for ions with large ionic radii and small radii of hydration.
- A proper buffer system must be selected in serological assays to ensure reliable results.
- The introduction of polymers can facilitate precipitation in a a secondary binding reactions because the presence of a polymer in a solution decreases the solubility of proteins. Linear hydrophilic polymers with high molecular weights are preferred.
13.2: Assays
Immunochromatographic Assays
These are rapid, specific, and sensitive and can be used in both laboratory and field tests for species identification.
Hexagon OBTI and ABAcard HemaTrace® can utilize the antibody–antigen–antibody sandwich method by using antibodies that recognize human Hb.
- ABAcard HemaTrace®: It utilizes a labeled monoclonal antihuman Hb antibody contained in a sample well, and a polyclonal antihuman Hb antibody immobilized at a test zone of a nitrocellulose membrane.
RSID™-Blood use antibodies that recognize human GPA.
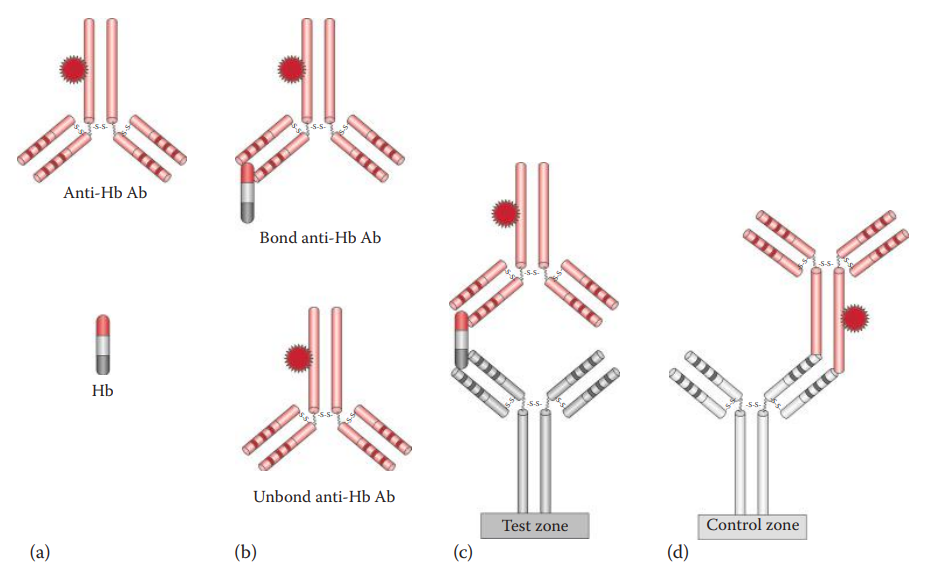
Double Immunodiffusion Assays
- Ring Assay: An antihuman antibody reagent is placed at the bottom of a test tube and a blood-stain extract is placed on top of the bottom layer.
- Ouchterlony Assay
- In a positive reaction, a line of precipitate will form between each antigen well and antibody well. This assay can also determine the similarity of the antigens.
- In an assay in which two antigens are loaded in adjacent wells and an antibody in the third well, the following results can be observed:
- Identity: A phenomenon wherein two antigens are identical, the two lines will become fused.
- Nonidentity: A phenomenon wherein two antigens are totally unrelated, the lines will cross each other but not fuse.
- Partial Identity: A phenomenon wherein the two antigens are related but are not identical, the lines will merge with spur formation.
Ring Assay Procedure
- Sample preparation and extraction
- Extract a portion of a stain with saline at 4°C overnight.
- Controls
- Include a positive control (known human serum sample) and a negative control (extraction blank).
- Loading of antibody and samples
- Spin the antihuman antibody in a microfuge and transfer the supernatant into test tubes or capillary tubes (depending on the volume of the stain and the antiserum extracted).
- Place the sample carefully over the top of the antiserum solution, which is usually denser than the sample.
- Immunodiffusion reaction
- Carry out the reaction at room temperature.
- In a positive reaction, white precipitate between the two layers can be observed after several minutes.
- This indicates that the sample is of human origin.
- No precipitate is formed if a bloodstain extract is from a nonhuman origin.
Ouchterlony Assay Procedure
- Sample preparation and extraction
- Cut out a small portion (approximately 5×5 mm) of a stain or a portion of a swab.
- Extract at room temperature in 100 μL of water for 30 min.
- The extract can be diluted if necessary.
- Alternatively, a very small piece of the stain or swab can be inserted directly into the well.
- Controls
- Positive (known serum)
- Negative (extraction blank)
- Substrate controls (extraction of substrate from unstained area) if applicable
- Agarose gel preparation
- Heat a suspension of agarose (4%) until liquefied.
- Cool the solution in a water bath at 55°C.
- Pour the agarose onto a piece of glass slide and let the gel solidify to a thickness of about 2–3 mm.
- Alternatively, a polyester support film such as GelBond (Cambrex, New Zealand) can be used as a gel support.
- The agarose should be poured onto the hydrophilic side of a piece of GelBond film (6×9 cm).
- Punch wells consisting of a central well surrounded by four wells using a template.
- Loading antibodies and samples
- Apply antihuman antibody to the central well. Apply the positive control to one of the surrounding wells.
- Apply the sample(s) in question next to a positive control.
- Apply negative and substrate controls to the remaining wells; only one negative control is needed per gel.
- Immunodiffusion reaction
- Incubate the plate overnight in a moisture chamber at 37°C.
- Staining
- Soak the gel overnight in saline solution and then soak it in deionized water for 10 min. Repeat once.
- Dry the gel between paper towels with a weight on top for 30 min.
- Dry in an oven for 30 min.
- Stain the gel with Coomassie blue. Stained precipitate bands appear blue.
Crossed-Over Electrophoresis
- This method is a combination of immunodiffusion and electrophoresis.
- A sharp precipitate band is visualized in a positive reaction.
- False-negative results can occur due to the post zone phenomenon, in which excess antigen may inhibit precipitation.
- False-negative results can also occur due to simple mistakes made during electrophoresis:
- Electrophoresis is carried out in the opposite direction, which results in samples running off the gel.
- Electrophoresis is carried out using an incorrect buffer system, affecting antigen–antibody binding.
Crossed-Over Electrophoresis Procedure
- Sample preparation and extraction
- Cut out a small portion (~5×5 mm) of a stain or a portion of a swab.
- Extract at room temperature in 100 μL of water for 30 min. The extract can be diluted if necessary.
- Alternatively, a very small piece of the stain or swab can be inserted directly into the well.
- Controls
- Positive (known serum)
- Negative (extraction blank)
- Substrate controls (extraction of substrate from unstained area) if applicable
- Agarose gel preparation
- Heat a suspension of agarose (4%) until liquefied.
- Cool the solution in a water bath at 55°C.
- Pour the agarose onto a piece of glass slide and let it solidify.
- Alternatively, a polyester support film such as GelBond can be used as a gel support.
- The agarose should be poured onto the hydrophilic side of a piece of GelBond (6×9 cm).
- Punch small wells (about 1–2 mm) in rows using a template.
- Loading antibodies and samples
- Apply antihuman antibody in one row of wells.
- Apply samples in the other row of wells.
- Apply the positive, negative, and substrate controls
- Electrophoresis
- Submerge the agarose gel in an electrophoresis tank in proper orientation.
- The wells containing antihuman antibody should be closest to the anode (positive electrode) and the wells containing samples should be closest to the cathode (negative electrode).
- During electrophoresis, the antibody in the antiserum should migrate toward the cathode while the antigen migrates toward the anode.
- Electrophoresis is carried out at 10 V/cm for 20 min.
- Staining Soak the gel overnight in a saline solution and then soak it in deionized water for 10 min. Repeat once.
- Dry the gel between paper towels with a weight on top for 30 min. Dry in an oven for 30 min.
- Stain the gel with Coomassie blue. Stained precipitate bands appear blue.