Important Thermodynamic Processes to Know for AP Physics 2 (2025)
Thermodynamic Basics
Thermodynamic processes describe how a system exchanges heat (Q), performs work (W), and experiences changes in internal energy (ΔU).
The First Law of Thermodynamics governs all processes:
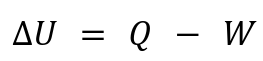
ΔU: Change in internal energy.
Q: Heat added to the system (positive if absorbed, negative if released).
W: Work done by the system (positive if the system expands, negative if compressed).
Thermodynamic processes play a critical role in systems like heat engines, refrigerators, and turbines, determining energy efficiency and system performance.
Thermodynamic Processes
1. Isothermal Process
Definition: The system's temperature (T) remains constant throughout the process.
Key Characteristics:
No change in internal energy:
ΔU = 0 (internal energy depends only on temperature for ideal gases).Heat added to the system is entirely converted into work:
Q = WRepresented on PV diagrams as a hyperbolic curve.
Work Done:
For an ideal gas, work is calculated as:
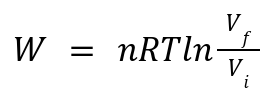
where Vf and Vi are final and initial volumes, respectively.
Applications:
Expansion or compression of gases in a slow process to maintain thermal equilibrium.
2. Adiabatic Process
Definition: No heat exchange occurs between the system and surroundings:
Q = 0Key Characteristics:
Changes in internal energy are due to work done on/by the system:
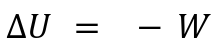
Temperature changes as work is done on/by the system.
Governed by the equation:
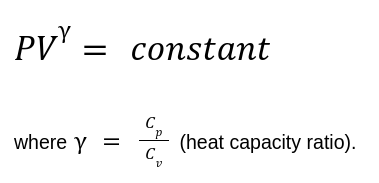
Work Done:
For an ideal gas:
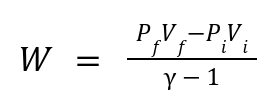
PV Diagram:
Curve is steeper than the isothermal curve due to the absence of heat exchange.
Applications:
Rapid compression/expansion processes, such as in engines or piston systems.
3. Isobaric Process
Definition: The process occurs at a constant pressure (P).
Key Characteristics:
Allows the system's volume to change while maintaining pressure.
Heat added or removed affects internal energy (ΔU) and enthalpy (ΔH).
Represented on a PV diagram as a horizontal line.
Work Done:
Work is calculated as:
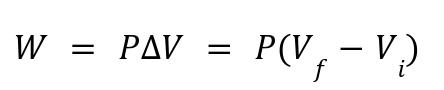
Applications:
Heating or cooling a gas in a container open to the atmosphere.
Example: Boiling water at atmospheric pressure.
4. Isochoric Process
Definition: The process occurs at a constant volume (V).
Key Characteristics:
No work is done since volume does not change:
W = 0.Any heat added (Q) changes the system's internal energy (ΔU):
ΔU = Q.Represented on PV diagrams as a vertical line.
Applications:
Heating a gas in a rigid, sealed container (e.g., a pressure cooker)
5. Cyclic Process
Definition: The system undergoes a series of processes and eventually returns to its initial state.
Key Characteristics:
Net change in internal energy (ΔU) is zero over the entire cycle.
The net work done (W) by the system is equal to the net heat added (Q).
Often visualized as a closed loop on a PV diagram.
Applications:
Heat engines (e.g., steam engines, internal combustion engines).
Refrigeration systems (reversing heat flow).
6. Isentropic Process
Definition: A reversible adiabatic process where entropy (S) remains constant.
Key Characteristics:
No heat exchange:

Changes in pressure, temperature, and volume are governed by adiabatic relationships.
Applications:
Idealized processes in turbines, compressors, and nozzles, aiming for maximum efficiency.
7. Throttling Process
Definition: A process where a fluid's pressure drops as it passes through a restriction or valve without heat exchange.
Key Characteristics:
No work is done on/by the system: W = 0, and Q = 0.
Temperature often decreases due to the Joule-Thomson effect.
Enthalpy remains constant:
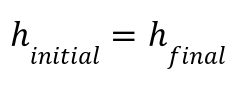
Applications:
Refrigeration cycles and gas expansion systems.
8. Polytropic Process
Definition: A generalized thermodynamic process that follows:
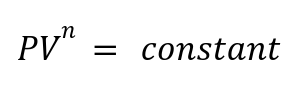
where n is the polytropic index.
Key Characteristics:
Can represent various processes depending on the value of n:
n = 0 : Isobaric process.
n = 1 : Isothermal process.
n = γ : Adiabatic process.
Models real gas behavior in engineering scenarios.
Applications:
Compression or expansion of gases in real-life systems.
Applications in Systems
1. Heat Engines and the Carnot Cycle
Heat engines convert thermal energy into mechanical work.
Operate between a hot reservoir (T_h) and a cold reservoir (T_c).
The Carnot cycle is an idealized model with maximum efficiency:
Isothermal expansion: Heat is absorbed from the hot reservoir.
Adiabatic expansion: System does work and cools without heat exchange.
Isothermal compression: Heat is released to the cold reservoir.
Adiabatic compression: System returns to its original state.
Maximum Efficiency:
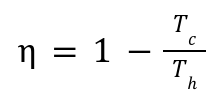
2. Refrigeration Cycles
Reverse of the Carnot cycle, used to cool systems:
Transfers heat from a low-temperature reservoir to a high-temperature reservoir.
Efficiency is measured using the Coefficient of Performance (COP):
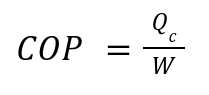
Qc: Heat removed from the cold reservoir.
W: Work input.
Applications: Refrigerators, air conditioners, heat pumps.