Conservation of Mass
Introduction
The objective of this lesson is to understand mass conservation in chemical reactions and calculate masses involved.
Law of Conservation of Mass
Definition: No atoms are lost or made during a chemical reaction, so the mass of the products equals the mass of the reactants.
Key Terms
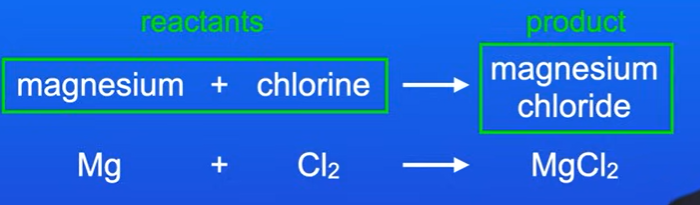
Reactants: Starting materials in a chemical reaction (e.g., magnesium and chlorine).
Products: Chemicals formed from a reaction (e.g., magnesium chloride).
Example Reaction
Magnesium and Chlorine Reaction
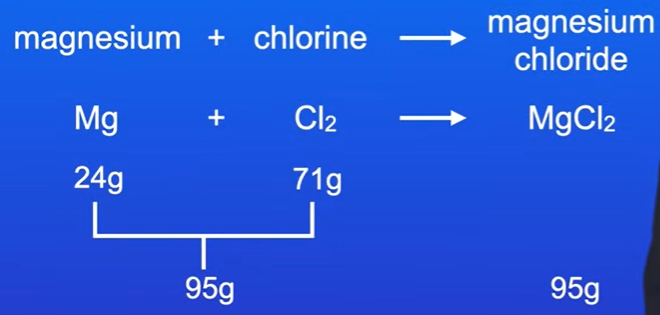
Chemical Equation: Magnesium + Chlorine → Magnesium Chloride
Given: 24 grams of magnesium and 71 grams of chlorine.
Calculating Mass of Products:
Total mass of reactants: 24g + 71g = 95g.
Mass of magnesium chloride produced = 95g.
Common Exam Question
Typical exam format involves calculating product mass using given reactant masses.
Practice Problems
Problem 1: Sodium and Oxygen Reaction
Given: 92 grams of sodium reacts with 32 grams of oxygen.
Calculation:
Total mass of reactants = 92g + 32g = 124g.
Mass of sodium oxide produced = 124g.
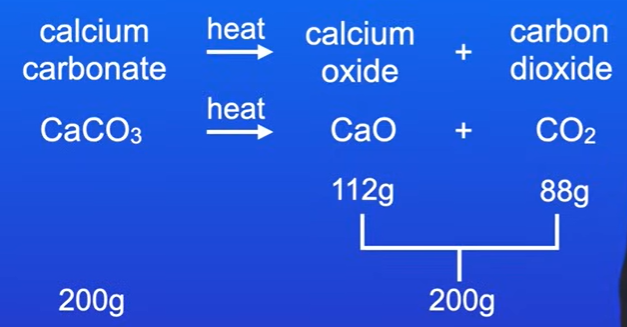
Problem 2: Calcium Carbonate Reaction
Given: Produces 112 grams of calcium oxide and 88 grams of carbon dioxide.
Task: Calculate mass of calcium carbonate that reacted.
Calculation:
Total mass of products = 112g + 88g = 200g.
Therefore, mass of calcium carbonate = 200g.
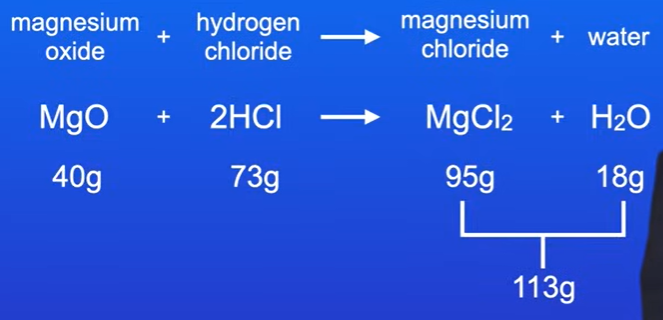
Problem 3: Magnesium Oxide Reaction
Given: Produces 95 grams of magnesium chloride and 18 grams of water with 73 grams of hydrogen chloride.
Calculation:
Total mass of products = 95g + 18g = 113g.
Mass of magnesium oxide = 113g - 73g = 40g.
Conclusion
Review and practice various mass calculation problems in chemistry.
Reference to Ovision workbook for additional practice questions.