biotech unit 2
organic molecules contain carbon
the major elements of life are carbon, hydrogen, nitrogen, and oxygen
and acronym for this is C.H.O.P.N.S
General tips
like dissolves like
Rubisco
makes inorganic Co2 into glucose
Carbon
VERY IMPORTANT TO BIOCHEM
can form up to four bond b/c of its number of valence electrons.
Covalent
shares electrons
ionic
takes away valence electron(s)
think of the time Mr Schuyler took Manav’s phone and ran around the room.
!!!STRUCTURE = FUNCTION!!!
Variation in Carbon skeletons
length
branching
sharing 2 valence electrons - double bonds(2 lines ==)
rings
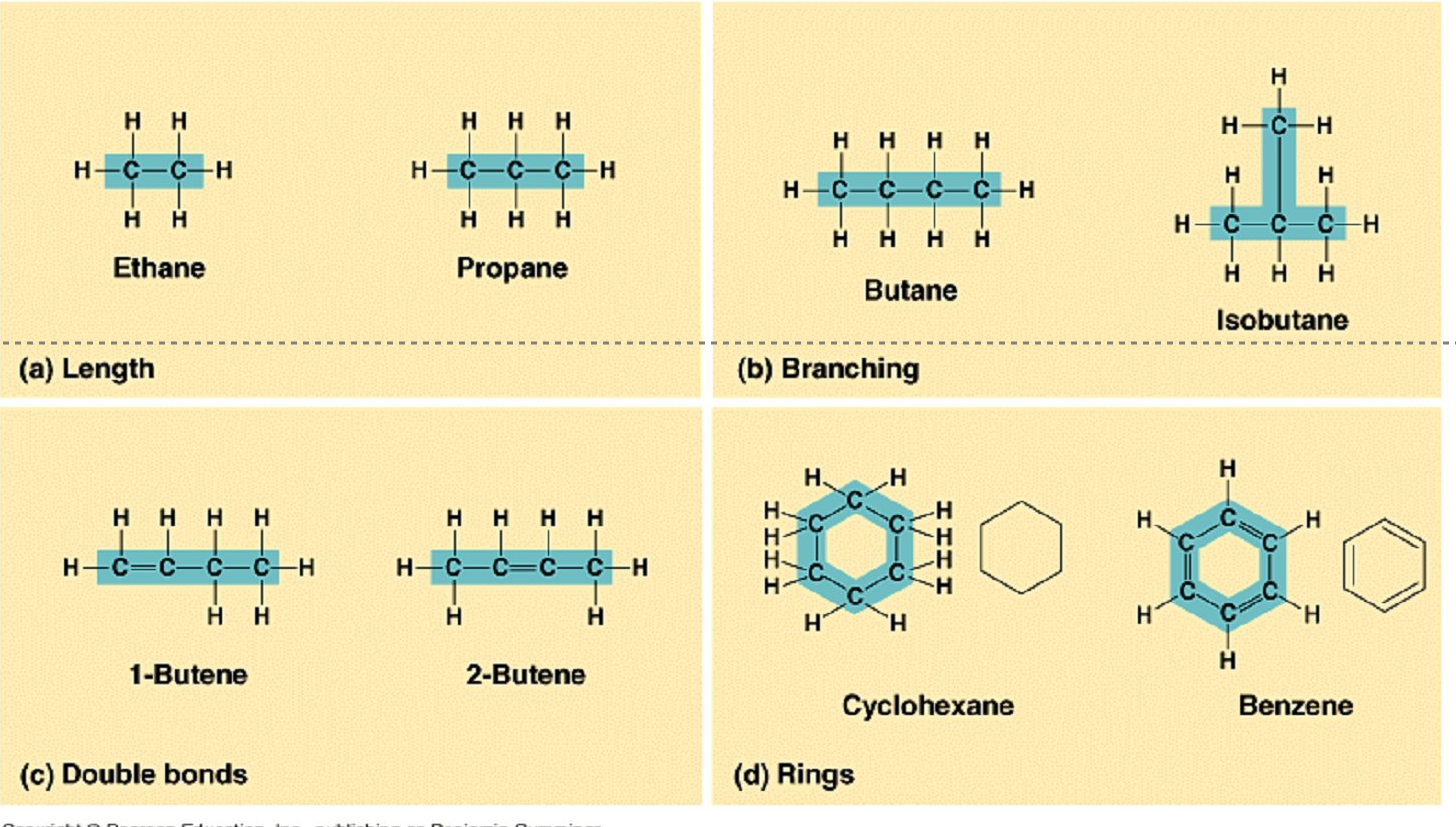
Hydrocarbons
Long C-H chain
non-polar covalent
Isomers
Compounds with the same formula, but a different structure
think of your 2 hands, and how they are reversed, but do the same thing
Hydrolysis(to break water, literally)
breakdown between monomers
removes water, then separates into a hydroxl(-OH) and an -H
digestive enzymes hydrolise food into monomers which then enter the bloodstream.
Dehydration synthesis
dehydration synthesis and hydrolysis have pretty much opposite names from their processes
makes larger covalent bonds that produce water
needs enzymes/catalyst.
Functional groups
behaves the same way regardless of molecule
effects the structure, therefore the function
Monomers
single building blocks
there are about 50 different monomers or”parts” that make up everything in our bodies, DNA included.
this makes us less complicated than cars
Carbohydrates
sugars are their polymers
dehydration synthesis - make a bigger molecule by removing water
classified based on numbers of simple sugars
Monosacharides
ratio of CH_2O, nutrients for cells(glucose) photosynthesis
energy in bonds
isomers
spatial arrangement - 3 d organization of molecules and organic compounds - CH_2O
forming in water
Disacharides
double sugar joined by glucose linkage(dehydration synthesis)
Polysacharides
100-1000 monosacharides
enzyme mediated dehydration synthesis
used for energy storage and structural support
Macromolecule
large organic polymer, has four classes
carbohydrates
lipids
proteins
nucleic acids
starch
short term energy
glucose polymer
storage in plants: t4 linkage(???)
granules in plastids( plastids are an organelle in plant cells that help with mitochondria)
amylose - simplest form - unbranched
amylopectin - branched form
Glycogen
storage in animals - large and highly branched
stored in muscle and liver - glucose polymer
Cellulose(structural)
linear chain of glucose units(Picture a straight, sugar necklace, the beads are glucose)
D-Glucose(in Humans)(just glucose needed to survive, standard glucose)
Chitin(structural)
polymer of amino sugar
exoskeleton in arthropods
Lipids
Think of oil
insoluble in water, but dissolves in non-polar solvents
fats, phospholipids(think the cell membranes Phospholipid bi-layer), and steroids
estrogen and testosterone are lipid based steroids.
Fats
2 parts - Glycerol(alcohol—> polar) and fatty acid
Glycerol - three carbon alcohol
fatty acid - carboxylic(fatty acid —>non polar)
16-18 carbons
non polar
enzyme linked dehydration synthesis
forms ester link(chemical bonds that join two parts of a molecule together)
do you see how the polar and the non polar come together????
Animal fats have no double bonds
vegetable fats can have double bonds, but they are loose(flexible connection)
Proteins
made of amino acids
four levels of protein structure
1) unique amino acid sequence
2)alpha helixes(twisty spiral staircase)(one strand)
3)hydrophobic interactions and disulfide bonds(defined somewhere here)
4)overall shape
central Dogma(DNA—> RNA—> Amino Acid—>Protein)
all enzymes are proteins, but not all proteins are enzymes(use da squares analogy)
Nucleic Acids
stores genetic material
genes
blueprint for building proteins
DNA—> RNA—>proteins
transfers information
blueprint for new cells and next gen
RNA is single helix
DNA is double helix
structure - monomers=nucleotide
Nucleic acids was the evidence darwin(or Lamark?) was missing!
Always remember the five prime three prime, if there are arrows pointing opposite directions, you can guess that.
Nucleotide
3 parts
nitrogen base(C-N ring)
pentose sugar(5 C)
ribose in RNA
Deoxyribose in DNA
phosphate(PO_4) group
Types of nucleotide
2 types
different nitrogen bases
purines
double ring N base
adenine
Guanine
“pure AG(silver)”
Pyrimidines
single ring N base
cytosine
thymine
uracil
Nucleic polymer
backbone
sugar to PO_4 bond
Phosphodiester bond(
new base added to sugar of previous base
polymer grows in one direction
N bases hang off the sugar-phosphate backbone
dangling bases are
Pairing Of Nucleotides
Nucleotides bond between DNA strands
H- bonds
purine::pyrimidine
A :: T
2 H bonds
G :: C
3 H bonds
DNA is a double helix
H bonds between bases and join the 2 strands
A :: T
C :: G
Copying DNA
replication
2 strands of DNA helix are complimentary
if it has one, it can build the other
if it has one, it can rebuild the whole
When does a cell copy DNA?
cell reproduction(mitosis)
gamete production(meiosis)
The History of DNA
Watson and Crick
“discovered” the structure of DNA, but used other peoples research such as . . .
Maurice Wilkins
discovered that A and T, G and C have the corresponding numbers
Watson and Crick assumed that meant they went together.
Rosalind Franklin
Saw the top of a DNA Helix
corrected Watson and crick on the order of the nucleic acid b/c they had it that the phosphate went on the inside, and that would explode.
moved back to France because she was sick of men
Information polymer
series of based encodes info like letters of a book(ttagcgtaccggcatccgat for example)
stored info is passed from parent to offspring(this needs to be copied accurately!!)
Gel electrophoresis
method used to separate particles by size
bigger molecules=less movement
DNA is negatively charged, so the wells start on the negative side and(run to red) and move towards the positive side(like how a magnet repels the same charge)
Hydroxl
_OH
alcohols(ethanol is an example)
polar
water soluble
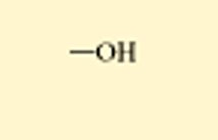
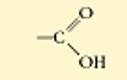
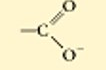
Carboxyl
the top is non ionized
the bottom is ionized
this functional group is acidic because it donates H- into the solution
polar
water soluble
a combo of a hydroxl and carbonyl
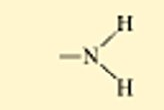
Amino group
the top is non ionized
the bottom is ionized
called amines
this group is a weak base because it gains a H+ ion, removing them from the solution(so the carboxyl is putting negative ions and Amino group is taking positive ions out of the solution)
non polar
generally water soluble
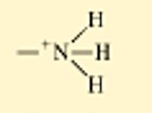
Sulfhydryl
called a thiol
helps stabilize protein structure(structure related to function!!) through disulfide bridges(2 sulfer atoms from different parts of the molecule bond very tightly)
polar
not very water soluble
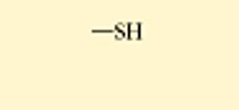
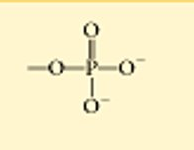
phosphate
called organic phosphates
acidic, looses h+ ions
polar
water soluble
unstable, so great for energy transfer and storage
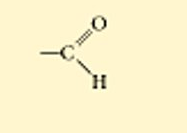
carbonyl
The top is an aldehyde, which means it is on the end of a molecule
the bottom is a ketone, which means it is in the middle of a molecule.
polar
water soluble
found in all sugars
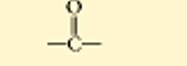 -
-